
Polymerisation of buta-1,3-diene by free radical mechanism gives _________.
A. trans-1,4-Polybutadiene
B. cis-1,4-Polybutadiene
C. Polyvinyl polythene
D. Polyallyl polythene
Answer
592.8k+ views
Hint: Polymerisation occurs through centres of radical formation. There are four radical centres in buta-1,3-diene as it has two double bonds present. The structure of buta-1,3-diene is
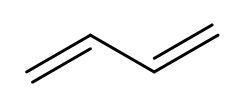
Just form the radicals and make the bonds between the radicals to produce three polymers of buta-1,3-diene.
Complete answer:
Free radical mechanism is a chain terminating type of polymerization. It occurs through formation of radicals at high temperature and pressure. Let us start forming the polymers.
The correct answer to this question is option ‘a’, ‘b’ and ‘c’.
Note: Do not form same type of polymers again and again by joining radicals formed at ${{\text{C}}_{1}}-{{\text{C}}_{2}}$ centre from the either side as the structure is symmetrical at ${{\text{C}}_{1}}-{{\text{C}}_{2}}$. So, be careful while forming polymers. The compounds formed will not be called as isomers as they are identical.

Just form the radicals and make the bonds between the radicals to produce three polymers of buta-1,3-diene.
Complete answer:
Free radical mechanism is a chain terminating type of polymerization. It occurs through formation of radicals at high temperature and pressure. Let us start forming the polymers.
| S. No. | Centres through which polymerization will take place | Mechanism of polymerization | Structures of polymers | Name of polymers |
| 1 | Carbon -1,2 or ${{\text{C}}_{1}}-{{\text{C}}_{2}}$ | In this, the double bond between ${{\text{C}}_{1}}-{{\text{C}}_{2}}$ will break into radicals and other molecules of buta-1,3-diene will also break from the same centre into radicals and then radicals of every other molecule will form single bond with each other and there is no breaking of another double bond. | 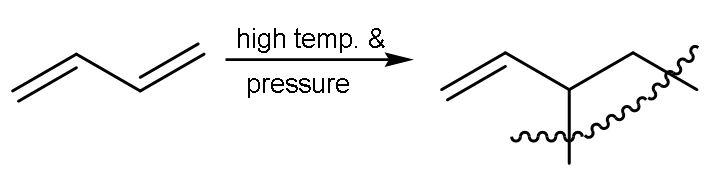
| The name of the polymer is polyvinyl polythene. |
| 2 | Carbon -1,4 or ${{\text{C}}_{1}}-{{\text{C}}_{4}}$ | In this, all the double bonds breaks into radicals and radicals between ${{\text{C}}_{1}}-{{\text{C}}_{4}}$ will form single bond with other molecules of buta-1,3-diene will also break from the same centre into radicals. But here, the radicals at ${{\text{C}}_{2}}-{{\text{C}}_{3}}$ join together to form a bond and single bond was already present so, double bond is made. The hydrogen atoms are on the same side on the double bond, so ‘cis’ polymer is formed. | 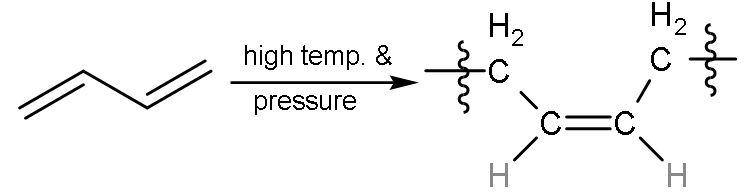
| The name of the polymer is cis-1,4-Polybutadiene. |
| 3 | Carbon -1,4 or ${{\text{C}}_{1}}-{{\text{C}}_{4}}$ | In this, all the double bonds breaks into radicals and radicals between ${{\text{C}}_{1}}-{{\text{C}}_{4}}$ will form single bond with other molecules of buta-1,3-diene will also break from the same centre into radicals. But here, the radicals at ${{\text{C}}_{2}}-{{\text{C}}_{3}}$ join together to form a bond and single bond was already present so, double bond is made. The hydrogen atoms are on the opposite side of the double bond, so a ‘trans’ polymer is formed. | 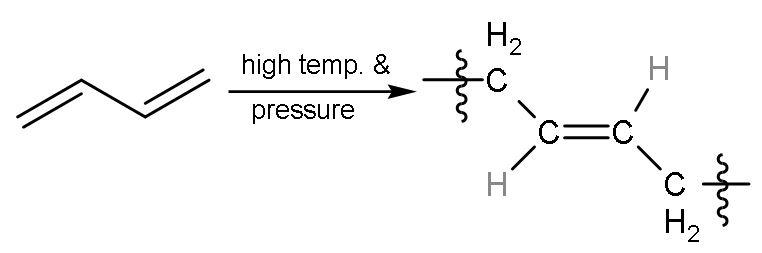
| The name of the polymer is trans-1,4-Polybutadiene. |
The correct answer to this question is option ‘a’, ‘b’ and ‘c’.
Note: Do not form same type of polymers again and again by joining radicals formed at ${{\text{C}}_{1}}-{{\text{C}}_{2}}$ centre from the either side as the structure is symmetrical at ${{\text{C}}_{1}}-{{\text{C}}_{2}}$. So, be careful while forming polymers. The compounds formed will not be called as isomers as they are identical.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




