
Potassium zincate is:
Answer
567k+ views
Hint: In a compound if there is Zn atom present as the central atom in the polyatomic anion then we name the anion as zincate.
- Zincates are generally formed by reaction with a solution of alkali.
Complete Solution :
- The branch of chemistry which deals with compounds other than carbon and hydrogen is called inorganic chemistry. In this branch of chemistry we deal with the composition, synthesis, extraction, structure and naming of these inorganic compounds.
- We know that for naming a compound, first we should know the anion and cation present. And we name the compound according to the cation and anions present in it. In some compounds cations and anions present may be polyatomic ones i.e. in them there will be a central ion and other atoms attached to the central atom and it exists as one unit.
- The question given is one example of such compounds.
Here in Potassium Zincate, Potassium is the cation and polyatomic anion is the zincate molecule.
Zincates is the name given as the suffix during the nomenclature of a polyatomic anion which possesses the Zn atom as the central atom in them.
- Zincates have a formulae of $ZnO_{2}^{2-}$ if prepared from the alkali salts
We know that generally zincates are prepared by dissolving zinc oxide or zinc ion in alkali solution.
- Now let’s write the formulae possible for the given compound.
In the lower classes we have studied the elements with their valency shown with respect to the valence electronic configuration.
Brushing up those memory we know that the, potassium ion (${{K}^{+}}$) possess a valency of +1 and the formulae for zincate is $ZnO_{2}^{2-}$ and it possess -2 valency.
By the method of crisscross we could easily find the formulae of potassium zincate.
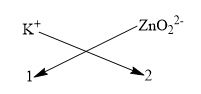
We get the formulae of potassium zincate as ${{K}_{2}}Zn{{O}_{2}}$.
Considering only the magnitude of the charge, the sign is not considered in the criss cross method for writing the formulae.
Note: Other units of zincates are $ZnCl_{4}^{2-}$, which is called as tetra chlorozincate ,$Zn(N{{O}_{3}})_{4}^{2-}$ which is tetra nitrite zincate.
- We should know the valence shown by each cation and anion to solve the problems related to writing the formulae of the compounds.
- Zincates are generally formed by reaction with a solution of alkali.
Complete Solution :
- The branch of chemistry which deals with compounds other than carbon and hydrogen is called inorganic chemistry. In this branch of chemistry we deal with the composition, synthesis, extraction, structure and naming of these inorganic compounds.
- We know that for naming a compound, first we should know the anion and cation present. And we name the compound according to the cation and anions present in it. In some compounds cations and anions present may be polyatomic ones i.e. in them there will be a central ion and other atoms attached to the central atom and it exists as one unit.
- The question given is one example of such compounds.
Here in Potassium Zincate, Potassium is the cation and polyatomic anion is the zincate molecule.
Zincates is the name given as the suffix during the nomenclature of a polyatomic anion which possesses the Zn atom as the central atom in them.
- Zincates have a formulae of $ZnO_{2}^{2-}$ if prepared from the alkali salts
We know that generally zincates are prepared by dissolving zinc oxide or zinc ion in alkali solution.
- Now let’s write the formulae possible for the given compound.
In the lower classes we have studied the elements with their valency shown with respect to the valence electronic configuration.
Brushing up those memory we know that the, potassium ion (${{K}^{+}}$) possess a valency of +1 and the formulae for zincate is $ZnO_{2}^{2-}$ and it possess -2 valency.
By the method of crisscross we could easily find the formulae of potassium zincate.

We get the formulae of potassium zincate as ${{K}_{2}}Zn{{O}_{2}}$.
Considering only the magnitude of the charge, the sign is not considered in the criss cross method for writing the formulae.
Note: Other units of zincates are $ZnCl_{4}^{2-}$, which is called as tetra chlorozincate ,$Zn(N{{O}_{3}})_{4}^{2-}$ which is tetra nitrite zincate.
- We should know the valence shown by each cation and anion to solve the problems related to writing the formulae of the compounds.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

State and prove converse of BPT Basic Proportionality class 10 maths CBSE




