
Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
(i)- 1-Bromo-1-methylcyclohexane
(ii)- 2-Chloro-2-methyl butane
(iii)- 2,2,3-Trimethyl-3-bromopentane
Answer
578.1k+ views
Hint: When haloalkane is reacted with sodium ethoxide, a molecule of hydrogen halide is eliminated and alkene is formed. It is a beta-elimination reaction. The product formed follows the Saytzeff rule.
Complete answer:
When a haloalkane is heated with sodium ethoxide, a molecule of hydrogen halide is eliminated and an alkene is formed. The hydrogen of the alkyl halide is eliminated from a beta-carbon and the halogen from the alpha-carbon. In some compounds there is the formation of two products, so to decide the major product, the Saytzeff rule is followed.
Syatzeff’s rule: If the halogen is present on any carbon atom within the chain, the alkyl halide can undergo dehydrohalogenation in two or more different directions depending upon the number of different types of \[\beta -hydrogen\] available. In all such cases, the more highly substituted alkene (having a lesser number of hydrogen atoms on the double-bonded carbon atoms) is the major product.
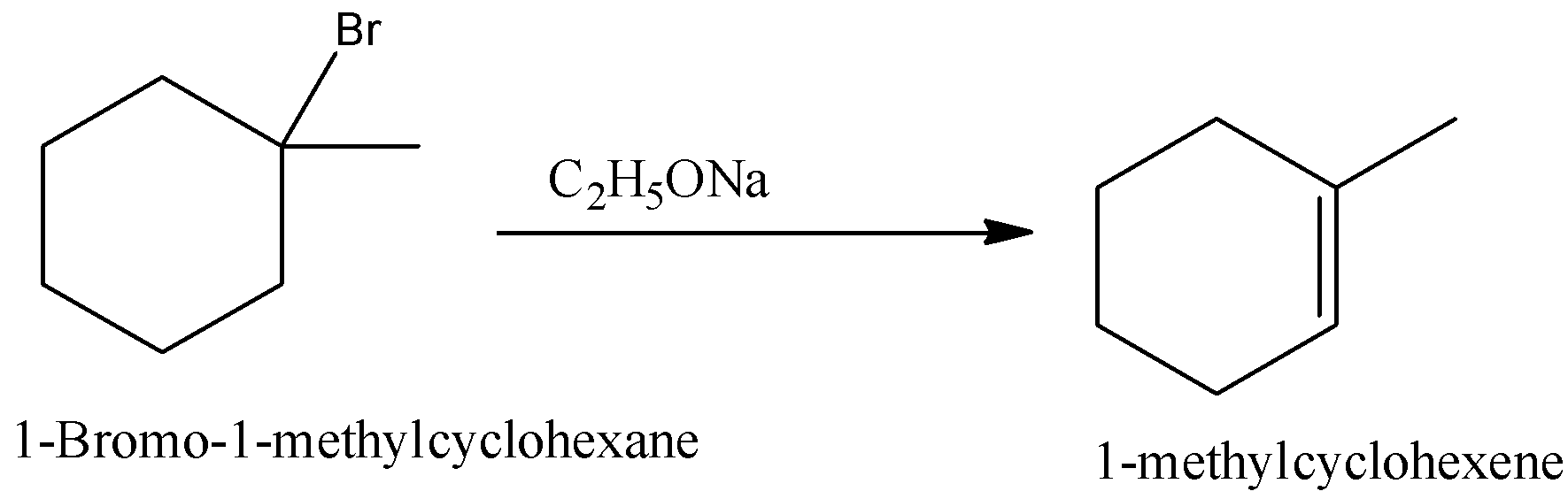
(i)- 1-Bromo-1-methylcyclohexane
When 1-Bromo-1-methylcyclohexane is reacted with sodium ethoxide, 1-methyl cyclohexene is formed. Beta-hydrogen is present at both equivalent sides, hence only one product is formed.
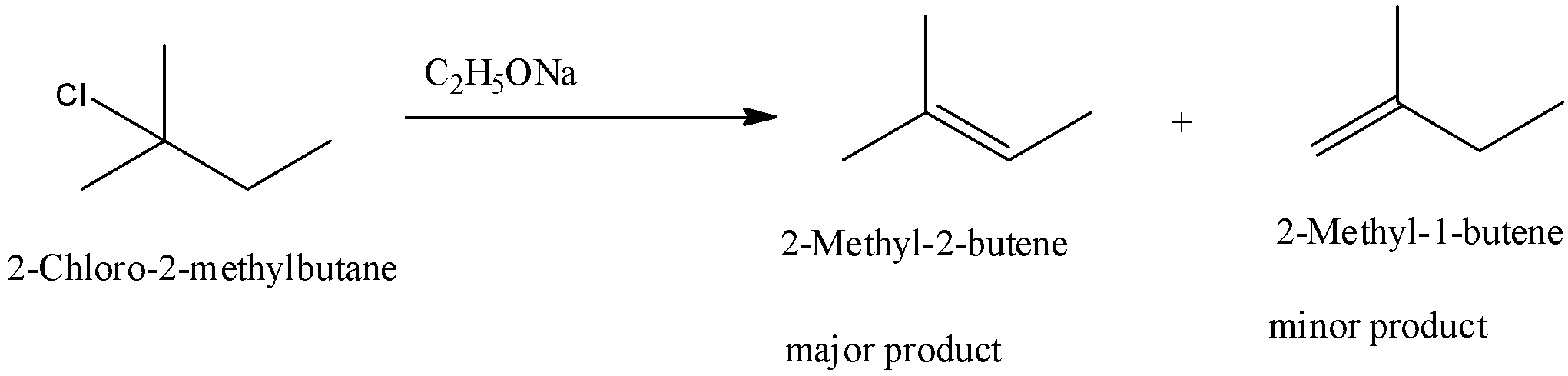
(ii)- 2-Chloro-2-methylbutane
When 2-Chloro-2-methyl butane is reacted with sodium ethoxide two products are formed.
2-Methyl-2-butene and 2-Methyl-1-butene are formed. The major product is 2-Methyl-2-butene because it is a more substituted product.
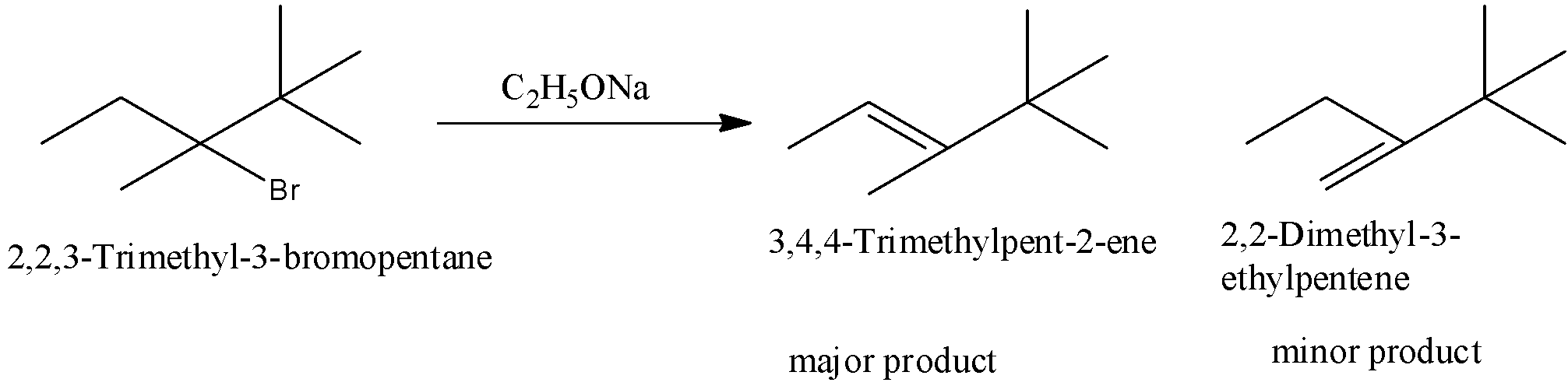
(iii)- 2,2,3-Trimethyl-3-bromopentane
When 2,2,3-Trimethyl-3-bromopentane is reacted with sodium ethoxide two products are formed. 3,4,4-Trimethylpent-2-ene and 2,2-Dimethyl-3-methylpentane. The major product is 3,4,4-Trimethylpent-2-ene because it is a more substituted product.
Note:
If the substituted alkene formed during Saytzeff elimination, is capable of showing cis-trans isomerism, the trans-alkene being more stable is always formed as the major product.
Complete answer:
When a haloalkane is heated with sodium ethoxide, a molecule of hydrogen halide is eliminated and an alkene is formed. The hydrogen of the alkyl halide is eliminated from a beta-carbon and the halogen from the alpha-carbon. In some compounds there is the formation of two products, so to decide the major product, the Saytzeff rule is followed.
Syatzeff’s rule: If the halogen is present on any carbon atom within the chain, the alkyl halide can undergo dehydrohalogenation in two or more different directions depending upon the number of different types of \[\beta -hydrogen\] available. In all such cases, the more highly substituted alkene (having a lesser number of hydrogen atoms on the double-bonded carbon atoms) is the major product.
(i)- 1-Bromo-1-methylcyclohexane
When 1-Bromo-1-methylcyclohexane is reacted with sodium ethoxide, 1-methyl cyclohexene is formed. Beta-hydrogen is present at both equivalent sides, hence only one product is formed.

(ii)- 2-Chloro-2-methylbutane
When 2-Chloro-2-methyl butane is reacted with sodium ethoxide two products are formed.
2-Methyl-2-butene and 2-Methyl-1-butene are formed. The major product is 2-Methyl-2-butene because it is a more substituted product.

(iii)- 2,2,3-Trimethyl-3-bromopentane
When 2,2,3-Trimethyl-3-bromopentane is reacted with sodium ethoxide two products are formed. 3,4,4-Trimethylpent-2-ene and 2,2-Dimethyl-3-methylpentane. The major product is 3,4,4-Trimethylpent-2-ene because it is a more substituted product.

Note:
If the substituted alkene formed during Saytzeff elimination, is capable of showing cis-trans isomerism, the trans-alkene being more stable is always formed as the major product.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




