
Predict the product $(T)$ for the following reaction sequence.
${{C}_{6}}{{H}_{5}}-CH=O\xrightarrow{KCN/O{{H}^{-}}}R\xrightarrow{Cr{{O}_{2}}/{{H}^{+}}}S\xrightarrow[(ii)Acidification]{(i)concKOH}T$ :
A. Cinnamic acid
B. Mandelic acid
C. Benzilic acid
D. Benzoic acid and Benzyl alcohol
Answer
554.1k+ views
Hint: in this question, benzil-benzilic acid rearrangement takes place. The acid formed in the given reaction is a white crystalline compound that is soluble in primary alcohols. it is hazardous to health causing redness and itching in the eye.
Complete step by step answer:.
This reaction is an example of benzil – benzilic acid rearrangement. Here, benzaldehyde reacts with $KCN$ in an alkaline medium and gives a product benzaldehyde cyanohydrin by converting one carbon into hydroxy group and the other into cyanide.
After this, chromium trioxide in acidic medium is used as a reagent for the oxidation. On oxidation, it gives benzil as a product.
After adding concentrated potassium hydroxide, one carbon at the centre is oxidised while the other carbon is reduced that results in the formation of benzilic acid.
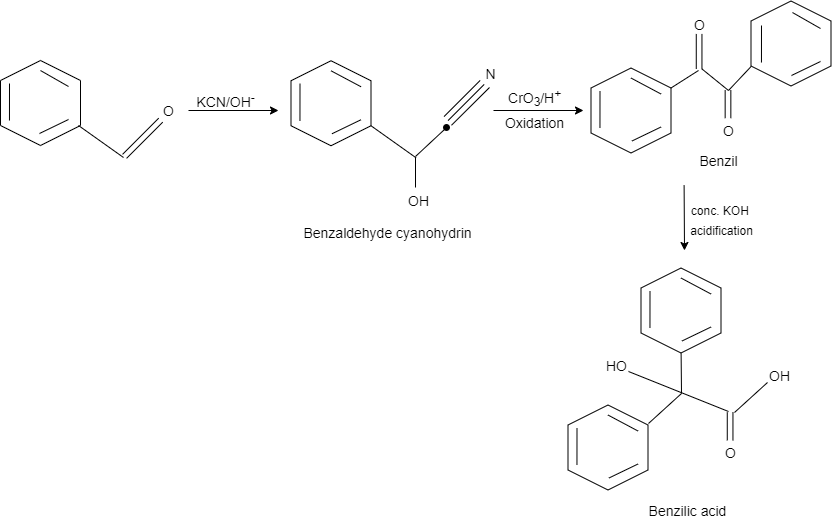
So, the correct answer is Option C.
Additional information :
The benzylic acid rearrangement is defined as the $1,2$ rearrangement of $1,2$ diketones, that gives a product, that is, $\alpha -$hydroxy carboxylic acid in which base is used as a rearrangement. This name is derived from the reaction of benzil with potassium hydroxide, that forms benzoic acid. It is viewed as an intramolecular disproportionation reaction, where one carbon is reduced while another carbon was oxidized.
Here, the hydroxide anion attacks on one of the keto groups in a nucleophilic addition reaction that gives alkoxide as a product. In the next step, it requires bond rotation to the conformer, which plays the migrating group $(R)$ in position for attack on the other carbonyl group. The migrating $R$ group attacks on $\alpha -$carbonyl group, that forms another alkoxide. This migration step is the rate determining step. The carboxylic acid in intermediate is less basic than alkoxide and hence reversible proton transfer takes place, which gives $\alpha -$hydroxy carboxylic acid.
Note: The reaction is a representative of 1,2-rearrangements. The long-established reaction mechanism was first proposed in its entirety by Christopher Kelk Ingold, and has been updated with silico data as outlined below. The reaction is second order overall in terms of rate, being first order in diketone and first order in base.
Complete step by step answer:.
This reaction is an example of benzil – benzilic acid rearrangement. Here, benzaldehyde reacts with $KCN$ in an alkaline medium and gives a product benzaldehyde cyanohydrin by converting one carbon into hydroxy group and the other into cyanide.
After this, chromium trioxide in acidic medium is used as a reagent for the oxidation. On oxidation, it gives benzil as a product.
After adding concentrated potassium hydroxide, one carbon at the centre is oxidised while the other carbon is reduced that results in the formation of benzilic acid.

So, the correct answer is Option C.
Additional information :
The benzylic acid rearrangement is defined as the $1,2$ rearrangement of $1,2$ diketones, that gives a product, that is, $\alpha -$hydroxy carboxylic acid in which base is used as a rearrangement. This name is derived from the reaction of benzil with potassium hydroxide, that forms benzoic acid. It is viewed as an intramolecular disproportionation reaction, where one carbon is reduced while another carbon was oxidized.
Here, the hydroxide anion attacks on one of the keto groups in a nucleophilic addition reaction that gives alkoxide as a product. In the next step, it requires bond rotation to the conformer, which plays the migrating group $(R)$ in position for attack on the other carbonyl group. The migrating $R$ group attacks on $\alpha -$carbonyl group, that forms another alkoxide. This migration step is the rate determining step. The carboxylic acid in intermediate is less basic than alkoxide and hence reversible proton transfer takes place, which gives $\alpha -$hydroxy carboxylic acid.
Note: The reaction is a representative of 1,2-rearrangements. The long-established reaction mechanism was first proposed in its entirety by Christopher Kelk Ingold, and has been updated with silico data as outlined below. The reaction is second order overall in terms of rate, being first order in diketone and first order in base.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




