
Preparation of benzene from phenol is:
(A) reduction
(B) oxidation
(C) addition
(D) dehydrogenation
Answer
586.2k+ views
Hint: Benzene is an organic chemical compound with the molecular formula ${{C}_ {6}} {{H}_ {6}} $The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. As it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon.
Complete step by step solution:
We have been provided with phenol,
Phenol is an aromatic organic compound with the molecular formula ${{C}_ {6}} {{H}_ {5}} OH$. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxyl group. Mildly acidic, it requires careful handling because it can cause chemical burns.
We need to prepare benzene from phenol,
For the preparation of benzene, we will be treating phenol with zinc:
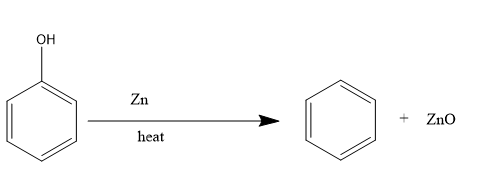
In the above reaction, phenol is being reduced with zinc to form benzene,
Reduction is a chemical reaction that involves the gaining of electrons by one of the atoms involved in the reaction between two chemicals. The term refers to the element that accepts electrons, as the oxidation state of the element that gains electrons is lowered.
So, we can say that preparation of benzene from phenol is reduced.
Therefore, we can say that option (A) is correct.
Note: Benzene is a widely used industrial chemical. Benzene is found in crude oil and is a major part of gasoline. It's used to make plastics, resins, synthetic fibres, rubber lubricants, dyes, detergents, drugs and pesticides. Benzene is produced naturally by volcanoes and forest fires.
Complete step by step solution:
We have been provided with phenol,
Phenol is an aromatic organic compound with the molecular formula ${{C}_ {6}} {{H}_ {5}} OH$. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (−C6H5) bonded to a hydroxyl group. Mildly acidic, it requires careful handling because it can cause chemical burns.
We need to prepare benzene from phenol,
For the preparation of benzene, we will be treating phenol with zinc:

In the above reaction, phenol is being reduced with zinc to form benzene,
Reduction is a chemical reaction that involves the gaining of electrons by one of the atoms involved in the reaction between two chemicals. The term refers to the element that accepts electrons, as the oxidation state of the element that gains electrons is lowered.
So, we can say that preparation of benzene from phenol is reduced.
Therefore, we can say that option (A) is correct.
Note: Benzene is a widely used industrial chemical. Benzene is found in crude oil and is a major part of gasoline. It's used to make plastics, resins, synthetic fibres, rubber lubricants, dyes, detergents, drugs and pesticides. Benzene is produced naturally by volcanoes and forest fires.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




