
How will you prepare ethanol, propan $ - 2 - $ ol and $2 - $ methyl propan $ - 2 - $ ol from Grignard’s reagent?
Answer
570.9k+ views
Hint: Primary alcohols are produced by the reaction of Grignard’s reagent with formaldehyde, followed by hydrolysis. Secondary alcohols are produced using aldehydes and tertiary alcohols using ketones, followed by hydrolysis. The number of carbon atoms in the product decides the type of compound used and the number of carbon atoms needed in the Grignard’s reagent.
Complete step by step answer:
Ethanol is a primary alcohol, and thus, can only be produced by reducing formaldehyde. Since formaldehyde has only one carbon atom, we should use a Grignard reagent with exactly one carbon atom to get ethanol (which has two carbon atoms), and follow it up with hydrolysis to get ethanol. Hence, we have:
$HCHO + C{H_3}MgBr\xrightarrow{{{H^ + }/{H_2}O}}C{H_3}C{H_2}OH + Mg(OH)Br$
For producing secondary and tertiary alcohols, we are free to vary the number of carbon atoms in both the Grignard reagent and the aldehyde/ketone. For ease of doing this problem, let us fix the Grignard reagent as methyl magnesium bromide ($C{H_3}MgBr$).
Propan $ - 2 - $ ol is a secondary alcohol with three carbon atoms. Hence, along with $C{H_3}MgBr$, we should use an aldehyde having two carbon atoms, that is, ethanal and follow it up with hydrolysis:
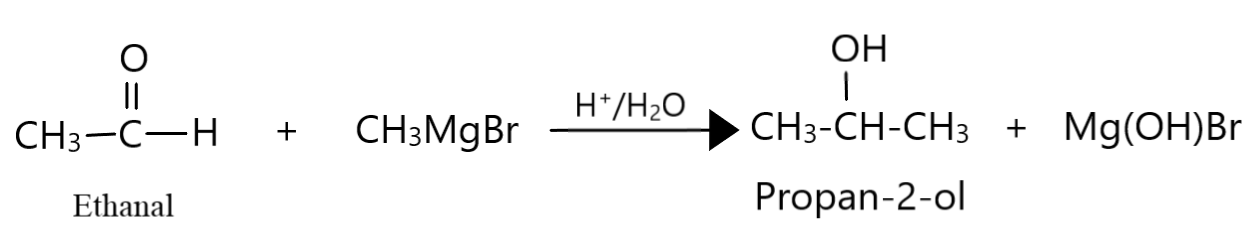
$2 - $ methyl propan $ - 2 - $ ol is a tertiary alcohol with four carbon atoms. Hence, along with $C{H_3}MgBr$, we have to use a ketone with three carbon atoms, that is, acetone and follow it up with hydrolysis:
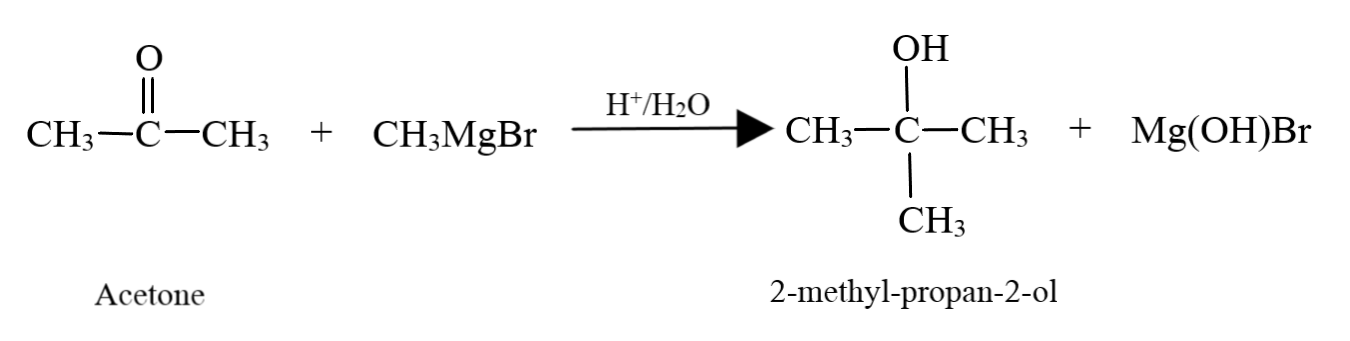
Hence, we have seen that the compounds needed to prepare ethanol, propan $ - 2 - $ ol and $2 - $ methyl propan $ - 2 - $ ol are: formaldehyde, ethanal and acetone respectively.
Note: Grignard reagents are organometallic halides, and are produced by treating alkyl halides with magnesium metal in the presence of dry ether. During their reduction of alcohols, the alkyl part acts as a nucleophile and attaches to the carbon atom having the carbonyl group. The magnesium halide portion gets attached to the oxygen and on hydrolysis, the corresponding alcohols are formed by the removal of the magnesium halide portion.
Complete step by step answer:
Ethanol is a primary alcohol, and thus, can only be produced by reducing formaldehyde. Since formaldehyde has only one carbon atom, we should use a Grignard reagent with exactly one carbon atom to get ethanol (which has two carbon atoms), and follow it up with hydrolysis to get ethanol. Hence, we have:
$HCHO + C{H_3}MgBr\xrightarrow{{{H^ + }/{H_2}O}}C{H_3}C{H_2}OH + Mg(OH)Br$
For producing secondary and tertiary alcohols, we are free to vary the number of carbon atoms in both the Grignard reagent and the aldehyde/ketone. For ease of doing this problem, let us fix the Grignard reagent as methyl magnesium bromide ($C{H_3}MgBr$).
Propan $ - 2 - $ ol is a secondary alcohol with three carbon atoms. Hence, along with $C{H_3}MgBr$, we should use an aldehyde having two carbon atoms, that is, ethanal and follow it up with hydrolysis:

$2 - $ methyl propan $ - 2 - $ ol is a tertiary alcohol with four carbon atoms. Hence, along with $C{H_3}MgBr$, we have to use a ketone with three carbon atoms, that is, acetone and follow it up with hydrolysis:

Hence, we have seen that the compounds needed to prepare ethanol, propan $ - 2 - $ ol and $2 - $ methyl propan $ - 2 - $ ol are: formaldehyde, ethanal and acetone respectively.
Note: Grignard reagents are organometallic halides, and are produced by treating alkyl halides with magnesium metal in the presence of dry ether. During their reduction of alcohols, the alkyl part acts as a nucleophile and attaches to the carbon atom having the carbonyl group. The magnesium halide portion gets attached to the oxygen and on hydrolysis, the corresponding alcohols are formed by the removal of the magnesium halide portion.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




