
Presence of excess sodium makes NaCl crystals coloured. Explain on the basis of crystal defects.
Answer
593.7k+ views
Hint: A compound is coloured due to the presence of electrons. In NaCl, due to a non-stoichiometric defect, an electron occupies an interstitial position and when it is excited, it imparts colour.
Complete answer:
As we know, solids are formed by a large network of crystals and this network is not always perfect. There are defects in these crystals which are generally irregular in arrangement of the constituents forming the network.
We can classify these defects as point defects which is the irregularity from the ideal arrangement around a point or an atom and line defects which is the irregularity in the entire row of the lattice.
Point defects are further classified into stoichiometry defects, impurity defects and non-stoichiometric defects. Sodium chloride can show all of these defects but the coloured crystals due to excess metal lies under non stoichiometric defects.
We can define the non-stoichiometry defects as the defects which contain elements in a non-stoichiometric ratio due to excess or deficiency of metal in the lattice.
When we heat the crystals of sodium chloride in presence of sodium vapour, sodium cations are deposited on the surface of the crystal. The chlorine anion diffuses to the surface of the crystal and combines with the sodium cation to give us sodium chloride. This happens due to the loss of electrons by sodium atoms to give us sodium cation. The released electron diffuses into the crystal lattice and occupies an anionic site which is called the F-centre. Due to this, the crystal has an excess of sodium. When this electron is excited, it imparts yellow colour, thus we see the crystals as yellow coloured.
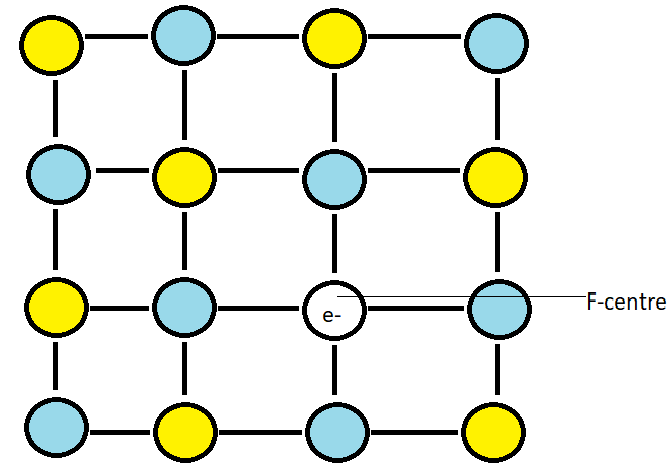
Note: Sodium chloride can have stoichiometric defects too but here, in the question it is mentioned to us that it has excess sodium ions therefore it cannot be a stoichiometric defect as excess sodium ions will change the stoichiometric ratio of sodium and chlorine which is 1:1 in NaCl. F-centres are also known as colour centres as the anionic site occupied by the electrons impart colour.
Complete answer:
As we know, solids are formed by a large network of crystals and this network is not always perfect. There are defects in these crystals which are generally irregular in arrangement of the constituents forming the network.
We can classify these defects as point defects which is the irregularity from the ideal arrangement around a point or an atom and line defects which is the irregularity in the entire row of the lattice.
Point defects are further classified into stoichiometry defects, impurity defects and non-stoichiometric defects. Sodium chloride can show all of these defects but the coloured crystals due to excess metal lies under non stoichiometric defects.
We can define the non-stoichiometry defects as the defects which contain elements in a non-stoichiometric ratio due to excess or deficiency of metal in the lattice.
When we heat the crystals of sodium chloride in presence of sodium vapour, sodium cations are deposited on the surface of the crystal. The chlorine anion diffuses to the surface of the crystal and combines with the sodium cation to give us sodium chloride. This happens due to the loss of electrons by sodium atoms to give us sodium cation. The released electron diffuses into the crystal lattice and occupies an anionic site which is called the F-centre. Due to this, the crystal has an excess of sodium. When this electron is excited, it imparts yellow colour, thus we see the crystals as yellow coloured.

Note: Sodium chloride can have stoichiometric defects too but here, in the question it is mentioned to us that it has excess sodium ions therefore it cannot be a stoichiometric defect as excess sodium ions will change the stoichiometric ratio of sodium and chlorine which is 1:1 in NaCl. F-centres are also known as colour centres as the anionic site occupied by the electrons impart colour.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




