
How many primary, secondary, tertiary, and quaternary carbon atoms are present in the following compound?
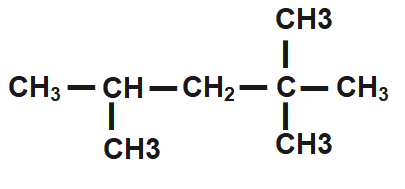
(A) One primary, two secondary and one tertiary.
(B) Five Primary, three secondary.
(C) Five primary, one secondary, one tertiary and one quaternary.
(D) Four primary, two secondary, and two quaternary.
Answer
537.9k+ views
Hint :The answer here is based on the concept of general chemistry that deals with the concept of the carbon atom that is bound to the number of hydrogen atoms in case of alkanes. Alkanes in which the carbon atom possesses three hydrogen atoms are called primary carbon atoms.
Complete Step By Step Answer:
The major concept of chemistry that includes the chapters based on the naming of the given compound by IUPAC rules, stability of carbocations, stability of carbanions and also primary carbons and much more are familiar to us. Now, let us see what it means by primary, secondary, and tertiary carbon of alkanes.
Alkanes in which the carbon atom is attached to two hydrogen atoms then that carbon is called secondary carbon and also this applies for alkenes as already there is presence of double bond between them and that particular carbon is attached to one hydrogen atom.
Finally, a carbon atom attached to only one hydrogen atom in case of alkanes is called tertiary carbon and here alkyne with here bonds between them and the carbon atom attached to one hydrogen atom will also be tertiary.
Thus, here are Five primary, one secondary, one tertiary and one quaternary.
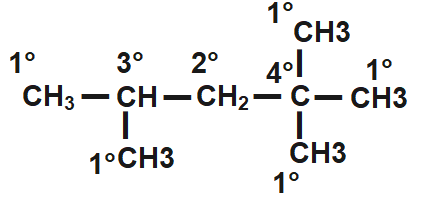
Therefore, correct answer is option C i.e. Five primary, one secondary, one tertiary and one quaternary.
Note :
Note that, in general, the carbon atom if attached to only on carbon atom then it is called primary carbon, if attached to two other carbons then it becomes secondary carbon and if attached to three other carbon atoms then it is called tertiary carbon and quaternary carbons one where the carbon is attached to four other carbon. This fact is important to note.
Complete Step By Step Answer:
The major concept of chemistry that includes the chapters based on the naming of the given compound by IUPAC rules, stability of carbocations, stability of carbanions and also primary carbons and much more are familiar to us. Now, let us see what it means by primary, secondary, and tertiary carbon of alkanes.
Alkanes in which the carbon atom is attached to two hydrogen atoms then that carbon is called secondary carbon and also this applies for alkenes as already there is presence of double bond between them and that particular carbon is attached to one hydrogen atom.
Finally, a carbon atom attached to only one hydrogen atom in case of alkanes is called tertiary carbon and here alkyne with here bonds between them and the carbon atom attached to one hydrogen atom will also be tertiary.
Thus, here are Five primary, one secondary, one tertiary and one quaternary.

Therefore, correct answer is option C i.e. Five primary, one secondary, one tertiary and one quaternary.
Note :
Note that, in general, the carbon atom if attached to only on carbon atom then it is called primary carbon, if attached to two other carbons then it becomes secondary carbon and if attached to three other carbon atoms then it is called tertiary carbon and quaternary carbons one where the carbon is attached to four other carbon. This fact is important to note.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




