
What is the product formed when \[o-\] Cresol reacts with bromine water?
A.
B.
C.
D.




Answer
547.5k+ views
Hint:\[o-\]cresol which is also known as $2-$ methyl phenol is a compound which has chemical formula $C{{H}_{3}}{{C}_{6}}{{H}_{4}}$ . It is used as an intermediate for the production of other chemical compounds. it is a colourless solid which is a derivative of phenol.
Complete step-by-step answer:Bromine water is a red yellow mixture that contains diatomic bromine which is dissolved in water. It can be prepared in a laboratory with mixing of fumes with water but it is not a safe method. Hence, a more convenient method is used for the preparation of bromine water solution. It is prepared by breaking down sodium bromide in presence of bleach and hydrochloric acid.
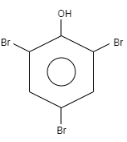
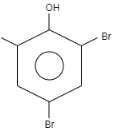
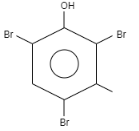
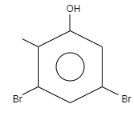
When \[o-\]Cresol is reacted with bromine water it results in the formation of ortho and Para bromo derivatives with respect to hydroxyl groups. It is a substitution reaction. In this reaction, the oxygen atom contains electrons, these electrons are donated by the oxygen to the single bond. At ortho position, the double bond of hydrogen replaces the bromine by attacking on diatomic bromine. It attacks the bromine and leaves the other bromine. Similarly at para position, the double bond of hydrogen replaces the bromine by attacking the diatomic bromine. It attacks the bromine and leaves the other bromine. This results in the formation of $2,4-$ dibromo $-6-$ methylphenol.
Let us see the reaction:
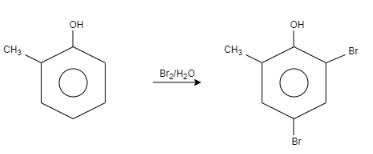
Note:In this question, we concluded that the halogenations reaction is a substitution reaction in which the halogen replaces one or more hydrogen of a compound. In this case, the bromine replaces the hydrogen of \[o-\] Cresol. Bromine water contains diatomic bromine dissolved in water.
Complete step-by-step answer:Bromine water is a red yellow mixture that contains diatomic bromine which is dissolved in water. It can be prepared in a laboratory with mixing of fumes with water but it is not a safe method. Hence, a more convenient method is used for the preparation of bromine water solution. It is prepared by breaking down sodium bromide in presence of bleach and hydrochloric acid.
When \[o-\]Cresol is reacted with bromine water it results in the formation of ortho and Para bromo derivatives with respect to hydroxyl groups. It is a substitution reaction. In this reaction, the oxygen atom contains electrons, these electrons are donated by the oxygen to the single bond. At ortho position, the double bond of hydrogen replaces the bromine by attacking on diatomic bromine. It attacks the bromine and leaves the other bromine. Similarly at para position, the double bond of hydrogen replaces the bromine by attacking the diatomic bromine. It attacks the bromine and leaves the other bromine. This results in the formation of $2,4-$ dibromo $-6-$ methylphenol.
Let us see the reaction:

Note:In this question, we concluded that the halogenations reaction is a substitution reaction in which the halogen replaces one or more hydrogen of a compound. In this case, the bromine replaces the hydrogen of \[o-\] Cresol. Bromine water contains diatomic bromine dissolved in water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




