
What is the product in the hydroxylation of $(z) - 3 - hexene$with $Os{O_4}$?
Answer
524.4k+ views
Hint : Hydroxylation is an oxidation reaction in which the carbon-hydrogen ($C - H$) bond is broken and replaced by the carbon–hydroxyl ($C - OH$) bond. The hydroxylation reaction is often mediated by catalysts and heat in organic chemistry. Metal ions are sometimes used as catalysts in hydroxylation reactions.
Complete Step By Step Answer:
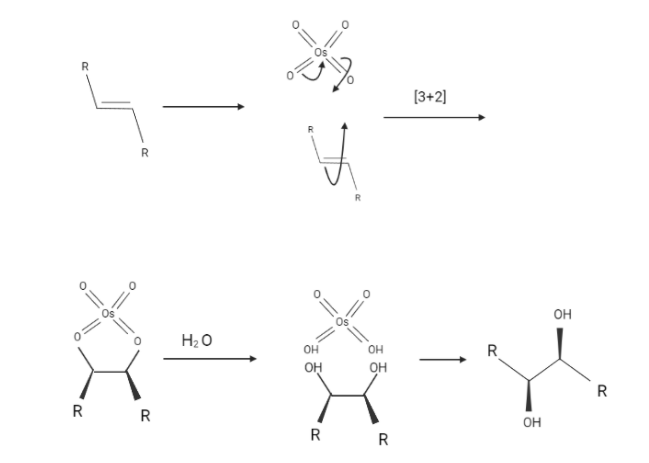
The $Os{O_4}$ reaction is a well-coordinated reaction with a cyclic intermediate and no rearrangements. Vicinal syn dihydroxylation is a step in the non dihydroxylation of an alkene that follows the epoxide-hydrolysis series. Stereocenters can form in the glycol substance when an alkene reacts with osmium tetroxide. Trans alkenes produce racemic mixtures, while cis alkenes produce meso materials.
Alkene dihydroxylation with OsO4 is a syn cycloaddition reaction that produces vicinal diols with cis stereochemistry.
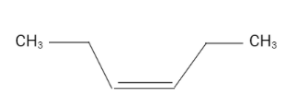
The structure of (Z)-hex-3-ene is
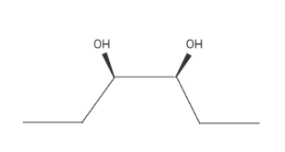
Because of the syn addition, all OH groups would be cis.
Note :
The reaction with alkenes has been altered due to the high cost and toxicity of Osmium tetroxide. To reduce some risks, catalytic concentrations of $Os{O_4}$ and stoichiometric amounts of an oxidizing agent such as hydrogen peroxide are still used. Also, potassium permanganate ($KMn{O_4}$), an older reagent, was used instead of $Os{O_4}$.
Complete Step By Step Answer:
The $Os{O_4}$ reaction is a well-coordinated reaction with a cyclic intermediate and no rearrangements. Vicinal syn dihydroxylation is a step in the non dihydroxylation of an alkene that follows the epoxide-hydrolysis series. Stereocenters can form in the glycol substance when an alkene reacts with osmium tetroxide. Trans alkenes produce racemic mixtures, while cis alkenes produce meso materials.
Alkene dihydroxylation with OsO4 is a syn cycloaddition reaction that produces vicinal diols with cis stereochemistry.

The structure of (Z)-hex-3-ene is

Because of the syn addition, all OH groups would be cis.

Note :
The reaction with alkenes has been altered due to the high cost and toxicity of Osmium tetroxide. To reduce some risks, catalytic concentrations of $Os{O_4}$ and stoichiometric amounts of an oxidizing agent such as hydrogen peroxide are still used. Also, potassium permanganate ($KMn{O_4}$), an older reagent, was used instead of $Os{O_4}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




