
What product is formed when acetic acid reacts with ${{P}_{2}}{{O}_{5}}$ ?
A. Acetyl chloride
B. Trichloro acetic acid
C. Acetic anhydride
D. Dichloro acetic acid
Answer
573.9k+ views
Hint: ${{P}_{2}}{{O}_{5}}$ called phosphorus pentoxide. It is a good dehydrating agent means it removes water very easily and the reaction is called dehydration reaction. The molecular formula of acetic acid is$C{{H}_{3}}COOH$ .
Complete Solution :
- In the question it is given what product we will get when acetic acid reacts with phosphorus pentoxide.
- The reaction of acetic acid with phosphorus pentoxide is as follows.
\[2C{{H}_{3}}COOH+{{P}_{2}}{{O}_{5}}\to {{(C{{H}_{3}}CO)}_{2}}O+{{H}_{2}}O\]
- In the above reaction two moles of acetic acid react with one mole of phosphorus pentoxide and form one mole of acetic anhydride and one mole of water as the products.
- Therefore acetic anhydride is going to form when acetic acid reacts with ${{P}_{2}}{{O}_{5}}$ .
- The structure of the acetic anhydride is as follows:
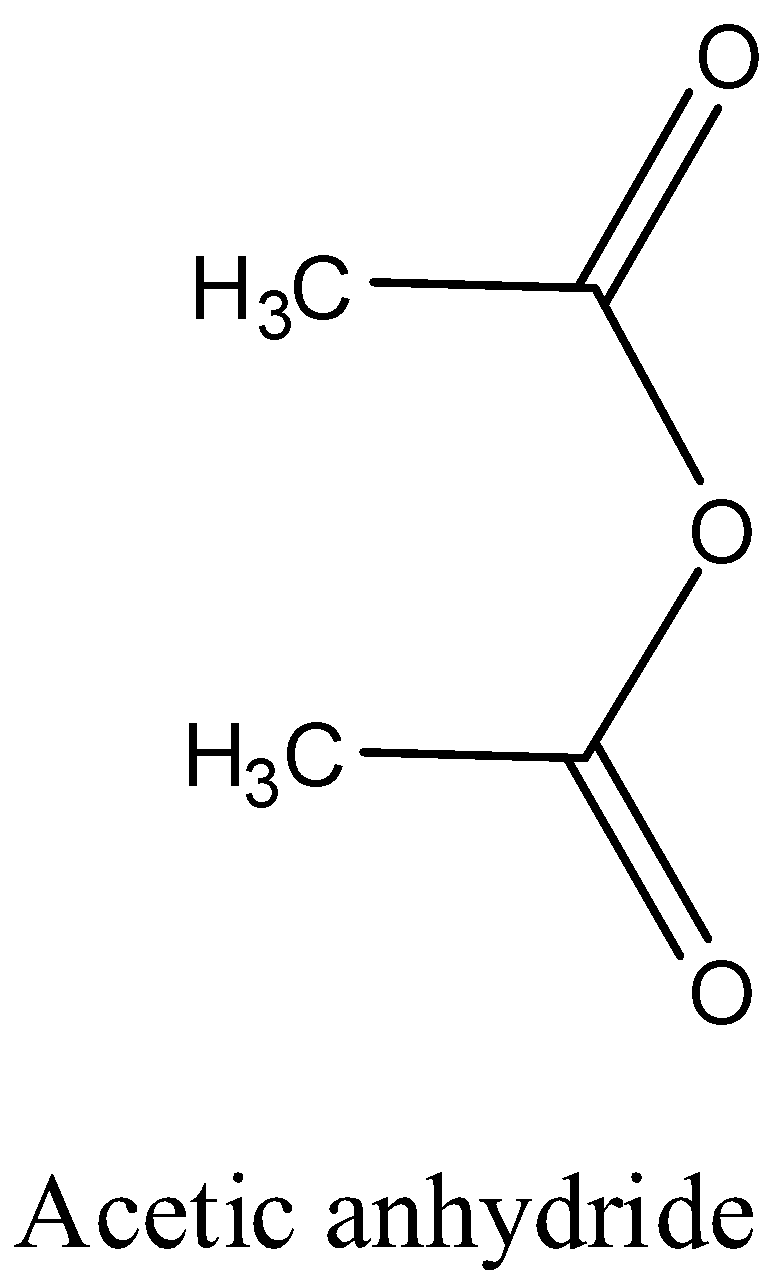
So, the correct answer is “Option A”.
Additional Information:
- The molecular formula of acetic anhydride is ${{C}_{4}}{{H}_{6}}{{O}_{3}}$ .
- Acetic acid also called as ethanoic acid.
- Acetic anhydride is commonly written as $A{{c}_{2}}O$ .
- Acetic anhydride is generally used in organic synthesis as an acetylating agent.
- In the preparation of aspirin acetic anhydride plays a crucial role.
Note: When we are going to add the acetic anhydride to the water again acetic acid is going to form and we can see the chemical reaction of acetic anhydride with water as follows:
\[{{(C{{H}_{3}}CO)}_{2}}O+{{H}_{2}}O\to 2C{{H}_{3}}COOH\]
One mole of acetic anhydride produces two moles of acetic acid and the above reaction is called hydration reaction.
Complete Solution :
- In the question it is given what product we will get when acetic acid reacts with phosphorus pentoxide.
- The reaction of acetic acid with phosphorus pentoxide is as follows.
\[2C{{H}_{3}}COOH+{{P}_{2}}{{O}_{5}}\to {{(C{{H}_{3}}CO)}_{2}}O+{{H}_{2}}O\]
- In the above reaction two moles of acetic acid react with one mole of phosphorus pentoxide and form one mole of acetic anhydride and one mole of water as the products.
- Therefore acetic anhydride is going to form when acetic acid reacts with ${{P}_{2}}{{O}_{5}}$ .
- The structure of the acetic anhydride is as follows:

So, the correct answer is “Option A”.
Additional Information:
- The molecular formula of acetic anhydride is ${{C}_{4}}{{H}_{6}}{{O}_{3}}$ .
- Acetic acid also called as ethanoic acid.
- Acetic anhydride is commonly written as $A{{c}_{2}}O$ .
- Acetic anhydride is generally used in organic synthesis as an acetylating agent.
- In the preparation of aspirin acetic anhydride plays a crucial role.
Note: When we are going to add the acetic anhydride to the water again acetic acid is going to form and we can see the chemical reaction of acetic anhydride with water as follows:
\[{{(C{{H}_{3}}CO)}_{2}}O+{{H}_{2}}O\to 2C{{H}_{3}}COOH\]
One mole of acetic anhydride produces two moles of acetic acid and the above reaction is called hydration reaction.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




