
What is the product of the reaction of \[1,3 - butadiene\] with \[B{r_2}\] ?
A. \[1,4 - dibromobut - 2 - ene\]
B. \[1,2 - dibromobutene\]
C. \[3,4 - dibromobutene\]
D. \[2,3 - dibromo - 2 - butene\]
Answer
493.2k+ views
Hint: Bromine shows a simple addition reaction with unsaturated organic compounds like alkenes and alkynes (due to their electron rich nature) where a double or triple gets broken and new carbon-bromine bonds are formed. But dienes are selective in nature and the bromine atoms attack at specific positions only.
Complete answer:
\[1,3 - butadiene\] is a conjugated diene i.e. its double bonds are alternatively placed and can therefore show resonance that adds to the stability of the compound but at the same time affects its reactivity.
Bromine is a monatomic molecule yet shows polarity. One of the double bonds of the conjugated diene gets attacked by electrophilic bromine atoms with partial positive charge so that the adjacent carbon becomes a stable conjugated carbocation.
The reaction proceeds in the direction in which a stable product is formed and not a stable intermediate. Though the formation of a secondary carbocation is more stable than a primary one, the nucleophilic bromine atom attacks the primary carbocation position as it results in a more stable alkene. (Based on Saytzeff’s rule that more substituted alkenes are more stable in nature).
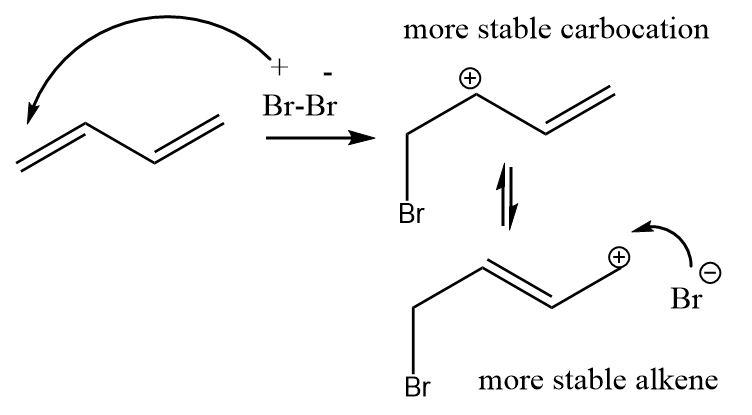
Thus, a \[1,4 - addition\] product called the thermodynamic product (in which a stable alkene is formed) is preferred over a \[1,2 - addition\] product called the kinetic product (in which a stable carbocation is formed).
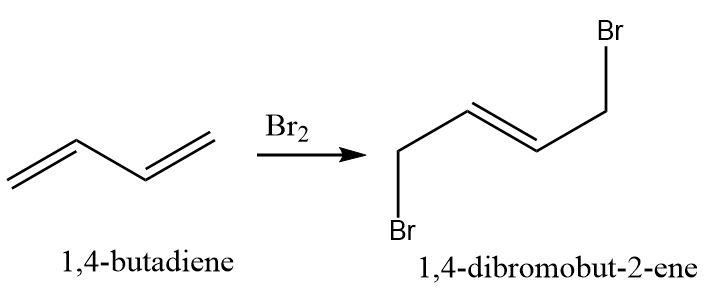
Hence, the correct option is (A)
Note:
The major product formed in the reaction is the thermodynamic product but on changing the reaction conditions a different product is obtained. If the same reaction is carried on at lower temperatures and in the presence of a polar solvent then the product obtained will be a \[1,2 - addition\] product i.e. \[3,4 - dibromobutene\].
Complete answer:
\[1,3 - butadiene\] is a conjugated diene i.e. its double bonds are alternatively placed and can therefore show resonance that adds to the stability of the compound but at the same time affects its reactivity.
Bromine is a monatomic molecule yet shows polarity. One of the double bonds of the conjugated diene gets attacked by electrophilic bromine atoms with partial positive charge so that the adjacent carbon becomes a stable conjugated carbocation.
The reaction proceeds in the direction in which a stable product is formed and not a stable intermediate. Though the formation of a secondary carbocation is more stable than a primary one, the nucleophilic bromine atom attacks the primary carbocation position as it results in a more stable alkene. (Based on Saytzeff’s rule that more substituted alkenes are more stable in nature).

Thus, a \[1,4 - addition\] product called the thermodynamic product (in which a stable alkene is formed) is preferred over a \[1,2 - addition\] product called the kinetic product (in which a stable carbocation is formed).

Hence, the correct option is (A)
Note:
The major product formed in the reaction is the thermodynamic product but on changing the reaction conditions a different product is obtained. If the same reaction is carried on at lower temperatures and in the presence of a polar solvent then the product obtained will be a \[1,2 - addition\] product i.e. \[3,4 - dibromobutene\].
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




