
What products are formed when the following compound is treated with $B{r_2}$ in the presence of $FeB{r_3}$ ?
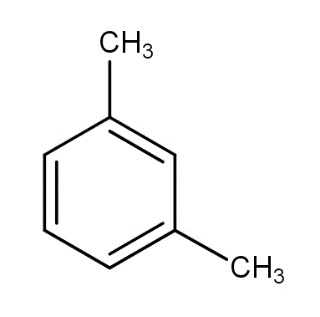
a.)
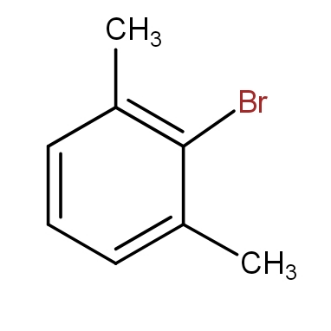 and
and
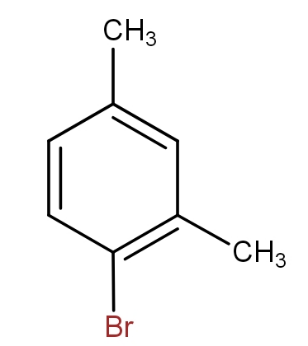
b.)
 and
and
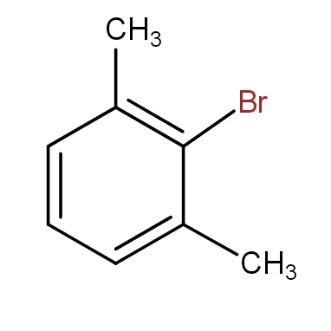
c.)
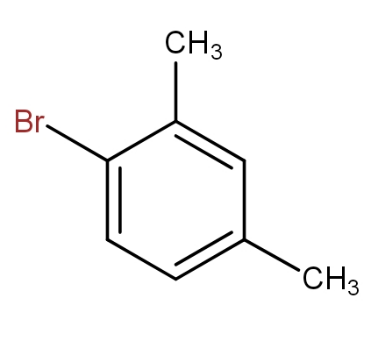 and
and
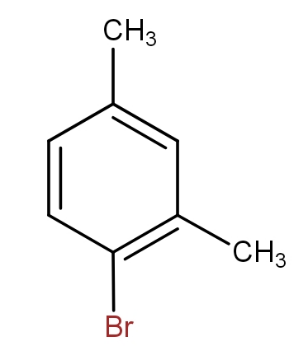
d.)
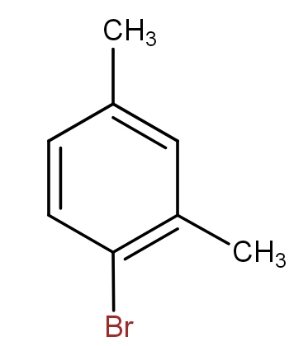 and
and
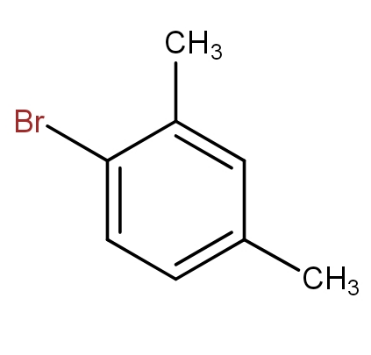









Answer
577.8k+ views
Hint: The meta xylene in the presence of ferric bromide reacts with bromine to give monosubstituted product. The methyl group is electron releasing in nature and is thus ortho para directing. So, it directs the incoming electrophile to ortho and para position.
Complete answer:
We have the substrate meta xylene. The reagents given to us give electrophilic substitution reactions. First, let us see what is an electrophilic substitution reaction.
The substitution reaction is one in which an atom is replaced by an incoming group. And when the incoming group is an electrophile, the reaction is called electrophilic substitution reaction.
In this reaction the Bromine atom is the electrophile and the meta xylene ring gets substituted.
We know that the methyl group is ortho para directing in nature. Thus, it will direct the incoming electrophile to ortho and para position. As, there are two methyl groups and both have equal effect, so a position which is ortho to one and para to another will get substituted. So, the reaction can be written as -
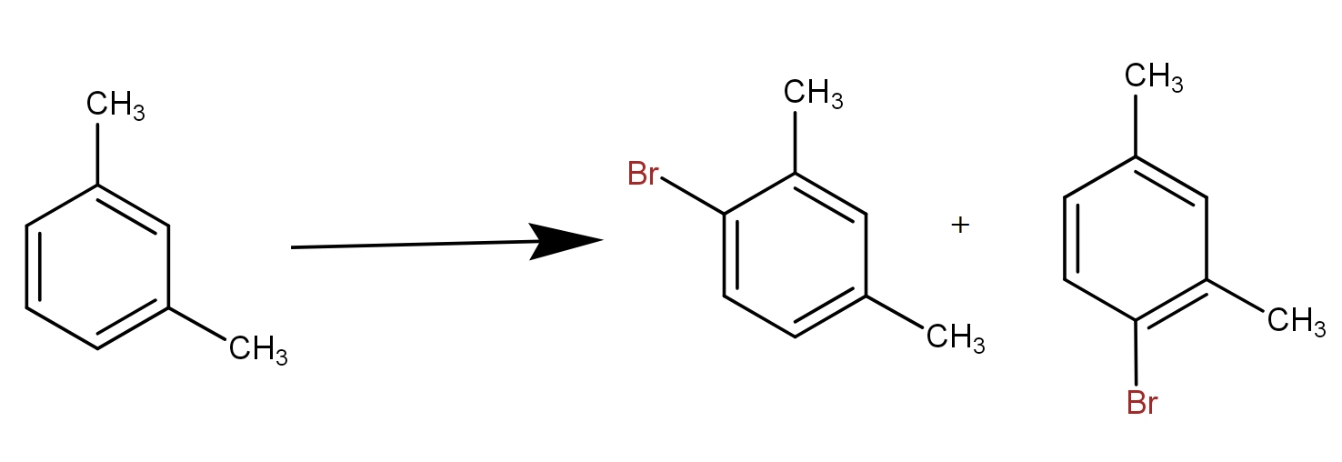
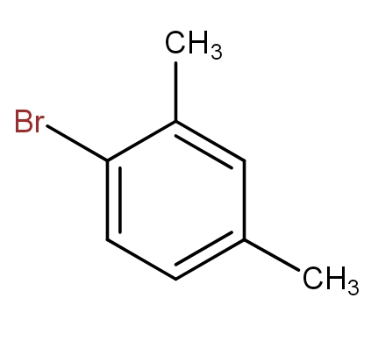
The meta xylene reacts with bromine in presence of ferric bromide to give 1 - bromo-2,4- dimethylbenzene. Thus, by seeing all the options, the option that contains the above combination is option d.).
So, the option d.) is the correct answer.
Note:
It must be noted that the product which is ortho to both the methyl groups can not be formed because of steric strain. The two methyl group presence meta to each other cause steric hindrance to the group that tries to attack at the carbon in between i.e. ortho to both.
Complete answer:
We have the substrate meta xylene. The reagents given to us give electrophilic substitution reactions. First, let us see what is an electrophilic substitution reaction.
The substitution reaction is one in which an atom is replaced by an incoming group. And when the incoming group is an electrophile, the reaction is called electrophilic substitution reaction.
In this reaction the Bromine atom is the electrophile and the meta xylene ring gets substituted.
We know that the methyl group is ortho para directing in nature. Thus, it will direct the incoming electrophile to ortho and para position. As, there are two methyl groups and both have equal effect, so a position which is ortho to one and para to another will get substituted. So, the reaction can be written as -

The meta xylene reacts with bromine in presence of ferric bromide to give 1 - bromo-2,4- dimethylbenzene. Thus, by seeing all the options, the option that contains the above combination is option d.).
So, the option d.) is the correct answer.
Note:
It must be noted that the product which is ortho to both the methyl groups can not be formed because of steric strain. The two methyl group presence meta to each other cause steric hindrance to the group that tries to attack at the carbon in between i.e. ortho to both.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




