
What is the propane gas chemical formula?
Answer
504.6k+ views
Hint: We can say that the chemical formula of a compound is a symbolic portrayal of its chemical composition. Chemical formulas give knowledge into the elements that comprise the particles/molecules of a compound and furthermore the proportion wherein the atoms of these components consolidate to give such molecules.
Complete answer:
We have to know that the chemical formula ' normally' alludes to the molecular formula of a compound. We can describe the compositions of chemical compounds in many ways. They are,
Molecular formula
Empirical formula
Structural formula
The molecular formula gives understanding into the quantity of components present in a compound. In molecular formulas, the components are signified by their particular symbols and the quantity of particles of every element in the particle is represented as subscript.
The empirical formula of a chemical formula addresses the proportion of the components present in that compound. Empirical formulae are typically acquired dependent on the examination of experimental information.
The structural formula of a substance compound gives understanding into the arrangements of the atoms present in the compound.
We know that propane is an alkane. Generally, alkanes have a chemical formula of ${C_n}{H_{2n + 2}}$. In propane, there are three carbon atoms present, so the value of n will be three.
Let us now substitute the value of n in the general formula of alkanes. So, ${C_3}{H_{2\left( 3 \right) + 2}}$ becomes ${C_3}{H_8}$. The chemical formula of propane is ${C_3}{H_8}$. The molecular formula of propane is also ${C_3}{H_8}$.
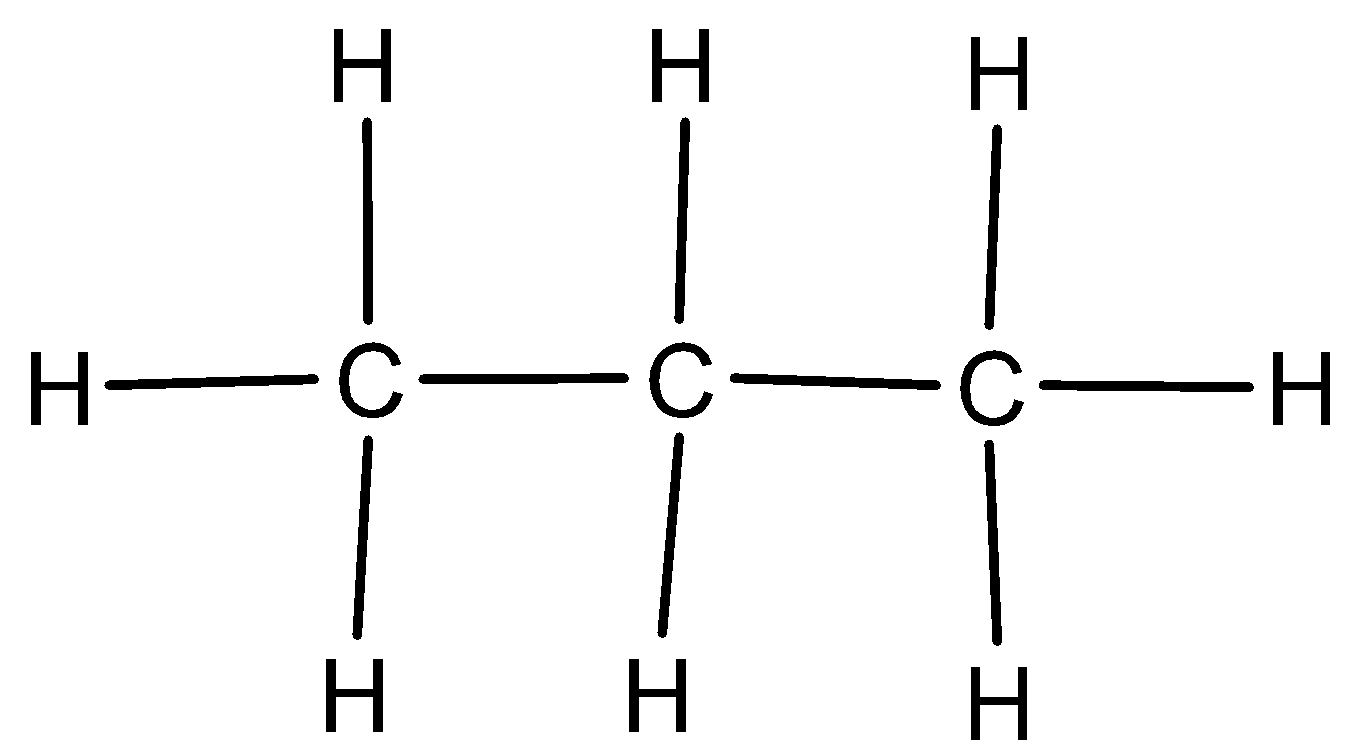
We can give the structural formula of propane as,
Note:
We can write the expanded chemical formula of propane as $C{H_3}C{H_2}C{H_2}$. One mole of propane has three atoms of carbons, and eight atoms of hydrogen. It is a colorless and odorless gas which is used in buses as biofuel, as major gas in blowtorches, and in hot air balloons.
Complete answer:
We have to know that the chemical formula ' normally' alludes to the molecular formula of a compound. We can describe the compositions of chemical compounds in many ways. They are,
Molecular formula
Empirical formula
Structural formula
The molecular formula gives understanding into the quantity of components present in a compound. In molecular formulas, the components are signified by their particular symbols and the quantity of particles of every element in the particle is represented as subscript.
The empirical formula of a chemical formula addresses the proportion of the components present in that compound. Empirical formulae are typically acquired dependent on the examination of experimental information.
The structural formula of a substance compound gives understanding into the arrangements of the atoms present in the compound.
We know that propane is an alkane. Generally, alkanes have a chemical formula of ${C_n}{H_{2n + 2}}$. In propane, there are three carbon atoms present, so the value of n will be three.
Let us now substitute the value of n in the general formula of alkanes. So, ${C_3}{H_{2\left( 3 \right) + 2}}$ becomes ${C_3}{H_8}$. The chemical formula of propane is ${C_3}{H_8}$. The molecular formula of propane is also ${C_3}{H_8}$.
We can give the structural formula of propane as,

Note:
We can write the expanded chemical formula of propane as $C{H_3}C{H_2}C{H_2}$. One mole of propane has three atoms of carbons, and eight atoms of hydrogen. It is a colorless and odorless gas which is used in buses as biofuel, as major gas in blowtorches, and in hot air balloons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




