
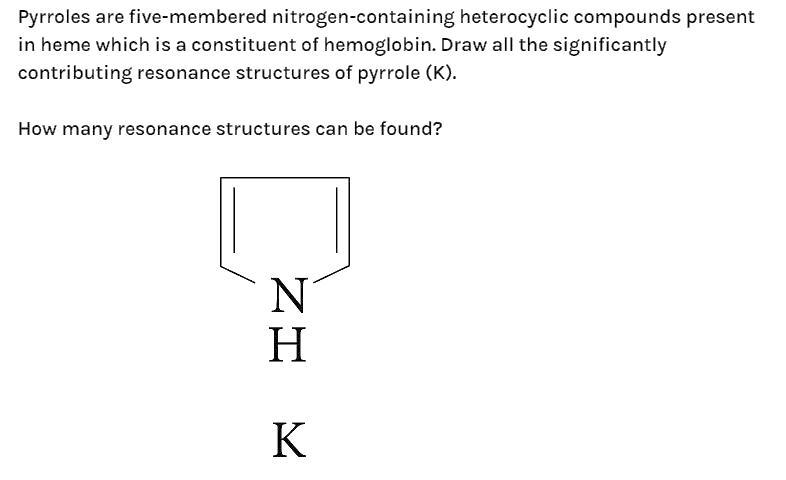
Pyrroles are five-membered nitrogen-containing heterocyclic compounds present in heme which is a constituent of haemoglobin. Draw all the significantly contributing resonance structures of pyrrole (K). How many resonance structures can be found?

Answer
600.6k+ views
Hint: We should understand that Pyrrole is a heterocyclic aromatic organic compound, a five-membered ring with the formula \[{{C}_{4}}{{H}_{4}}NH\]. Resonance structures should have the same number of electrons, do not add or subtract any electrons. We should draw a valid Lewis structure. Then we should move a π electron pair in a double bond and then adjust the formal charges on atoms. Repeat for every additional double bond in the system.
Step by step answer:
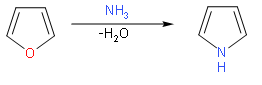
Pyrrole is prepared industrially by treatment of furan with ammonia in the presence of solid acid catalysts, like \[Si{{O}_{2}}\] and\[A{{l}_{2}}{{O}_{3}}\].
Before drawing resonance structures of pyrrole, we should first know about some rules that we have to follow in drawing resonance structures: we should know that in resonance structures, the electrons are able to move to help stabilize the molecule. This movement of the electrons is called delocalization
We should know that resonance structures should have the same number of electrons, do not add or subtract any electrons. (Check the number of electrons by simply counting them).
All resonance structures must follow the rules of writing Lewis Structures.
The hybridization of the structure must stay the same.
The skeleton of the structure cannot be changed (only the electrons move).
Resonance structures must also have the same amount of lone pairs.
We can make four resonance structures of pyrrole.
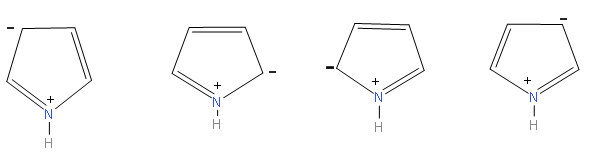
By observing the above structures we can say that, there are a total four resonance structures of pyrrole.
Additional information:
We should understand that Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of natural products. Common naturally produced molecules containing pyrroles include vitamin B12, bile pigments like bilirubin, chlorophyll.
We identify Pyrrole as a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. If we try to smell it, a nut-like smell comes from it. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene.
Note: In chemistry resonance structures are very important. We should note that resonance is a way of describing bonding in certain molecules or ions by the combination of several contributing structures. Resonance is significant for us to understand because it has particular value for describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
Step by step answer:
Pyrrole is prepared industrially by treatment of furan with ammonia in the presence of solid acid catalysts, like \[Si{{O}_{2}}\] and\[A{{l}_{2}}{{O}_{3}}\].

Before drawing resonance structures of pyrrole, we should first know about some rules that we have to follow in drawing resonance structures: we should know that in resonance structures, the electrons are able to move to help stabilize the molecule. This movement of the electrons is called delocalization
We should know that resonance structures should have the same number of electrons, do not add or subtract any electrons. (Check the number of electrons by simply counting them).
All resonance structures must follow the rules of writing Lewis Structures.
The hybridization of the structure must stay the same.
The skeleton of the structure cannot be changed (only the electrons move).
Resonance structures must also have the same amount of lone pairs.
We can make four resonance structures of pyrrole.

By observing the above structures we can say that, there are a total four resonance structures of pyrrole.
Additional information:
We should understand that Pyrrole itself is not naturally occurring, but many of its derivatives are found in a variety of natural products. Common naturally produced molecules containing pyrroles include vitamin B12, bile pigments like bilirubin, chlorophyll.
We identify Pyrrole as a colorless volatile liquid that darkens readily upon exposure to air, and is usually purified by distillation immediately before use. If we try to smell it, a nut-like smell comes from it. Pyrrole is a 5-membered aromatic heterocycle, like furan and thiophene.
Note: In chemistry resonance structures are very important. We should note that resonance is a way of describing bonding in certain molecules or ions by the combination of several contributing structures. Resonance is significant for us to understand because it has particular value for describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




