
What is the radius of the iodine atom (atomic number is given as $53$, mass number is given as $126$)?
$\begin{align}
& A.2.5\times {{10}^{-11}}m \\
& B.2.5\times {{10}^{-9}}m \\
& C.7\times {{10}^{-9}}m \\
& D.7\times {{10}^{-6}}m \\
\end{align}$
Answer
584.4k+ views
Hint: The radius of this atom can be found by taking the ratio of the product of the radius of the hydrogen atom and the square of the number of shells of the iodine atom to the atomic number of the iodine atom. The electronic configuration of the atom is to be taken in order to find the number of shells. These all may help you to solve this question.
Complete Answer:
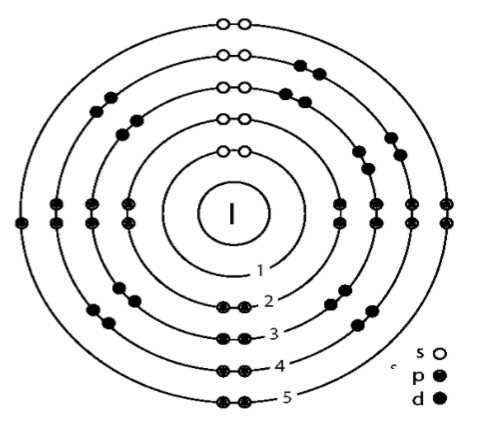
The electronic configuration of the iodine atom can be found at first. The atomic number is given as
$Z=53$
Therefore the electronic configuration will be written as,
$2,8,18,18,7$
From this we can see that the number of shells can be calculated.
That is,
$n=5$
The radius of an atom can be calculated by an equation given as,
${{r}_{n}}={{r}_{H}}\dfrac{{{n}^{2}}}{Z}$
Where ${{r}_{H}}$be the radius of the hydrogen atom, $Z$be the atomic number and $n$ be the number of shells.
The radius of hydrogen atom is given as,
${{r}_{H}}=0.053\times {{10}^{-9}}m$
The atomic number of the iodine atom is given as,
$Z=53$
And the number of shells in the iodine atom is already given as,
\[n=5\]
Substituting the values in it will give,
\[{{r}_{n}}=\left( 0.053\times {{10}^{-9}} \right)\dfrac{{{5}^{2}}}{53}\]
Simplifying the equation will give the radius of iodine atom,
\[{{r}_{n}}=2.5\times {{10}^{-11}}m\]
Therefore the correct answer has been obtained.
The answer is given as option A.
Note:
Iodine is an element in the periodic table which is having the symbol \[I\]. Iodine is helpful in the treatment and prevention of the iodine deficiency and is also used as an antiseptic. Iodine is the least abundant among the halogen gases. And also it is the heaviest mineral nutrient. Radioactive isotopes of the iodine can be used to treat thyroid cancer.
Complete Answer:
The electronic configuration of the iodine atom can be found at first. The atomic number is given as
$Z=53$
Therefore the electronic configuration will be written as,
$2,8,18,18,7$
From this we can see that the number of shells can be calculated.
That is,
$n=5$
The radius of an atom can be calculated by an equation given as,
${{r}_{n}}={{r}_{H}}\dfrac{{{n}^{2}}}{Z}$
Where ${{r}_{H}}$be the radius of the hydrogen atom, $Z$be the atomic number and $n$ be the number of shells.
The radius of hydrogen atom is given as,
${{r}_{H}}=0.053\times {{10}^{-9}}m$
The atomic number of the iodine atom is given as,
$Z=53$
And the number of shells in the iodine atom is already given as,
\[n=5\]
Substituting the values in it will give,
\[{{r}_{n}}=\left( 0.053\times {{10}^{-9}} \right)\dfrac{{{5}^{2}}}{53}\]
Simplifying the equation will give the radius of iodine atom,
\[{{r}_{n}}=2.5\times {{10}^{-11}}m\]
Therefore the correct answer has been obtained.
The answer is given as option A.

Note:
Iodine is an element in the periodic table which is having the symbol \[I\]. Iodine is helpful in the treatment and prevention of the iodine deficiency and is also used as an antiseptic. Iodine is the least abundant among the halogen gases. And also it is the heaviest mineral nutrient. Radioactive isotopes of the iodine can be used to treat thyroid cancer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




