
Rank the following alkenes in order of decreasing heat of hydrogenation (largest first):
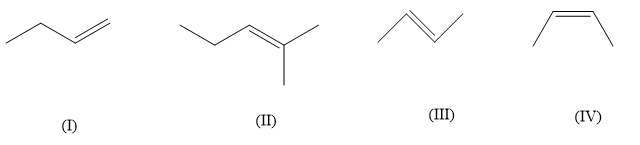
(A) $ II > III > IV > I $
(B) $ II > IV > III > I $
(C) $ I > III > IV > II $
(D) $ I > IV > III > II $

Answer
546.3k+ views
Hint: The more stable the alkene will be lower will be its heat of hydrogenation. Substitution on the alkene stabilizes them and trans forms are stable than respective cis form.
Complete Step by step answer:
Heat of hydrogenation is the heat involved in the reaction when an alkene undergoes a reaction with $ {H_2} $ to give a saturated product. Now the heat of hydrogenation is usually negative and is dependent on the groups and geometry of the alkene. In the given alkenes the number of alkyl groups are different so their heat of hydrogenation will also be different. Also for same compound having two different geometrical isomers cis and trans, the hydrogenation energy will be different. For a given alkene more the alkyl groups or other substituents are attached, lesser will be the heat of hydrogenation. Lesser heat of hydrogenation means that the bonds are stronger and more stable, which means alkyl and other substitution on alkenes increases their stability. So, in the above compound (II) compound has the highest substitution of three alkyl groups and (I) has the lowest alkyl substitution of only one alkyl group whereas, compound (III)&(IV) both have two alkyl substitution. So now we can’t say about (III)&(IV) which one will have lower heat of hydrogenation. In case of cis and trans isomers, the trans isomers evolve less heat, this is because the cis isomers are more crowded due to non-bonded interactions between two alkyl groups on the same side of the double bond. So, compound (IV) is cis-butene whereas compound (III) is trans-butene, which means that compound (III) will have lower heat of hydrogenation. So, compound (II) will have the lowest heat of hydrogenation followed by compound (III), (IV) and (I).
So, the option will be (d).
Note:
Other than synthetic applications, catalytic hydrogenation is useful for analytical and thermochemical purposes. The amount of uptake of hydrogens by an unknown compound can give us an estimation about the unsaturation present in the system.
Complete Step by step answer:
Heat of hydrogenation is the heat involved in the reaction when an alkene undergoes a reaction with $ {H_2} $ to give a saturated product. Now the heat of hydrogenation is usually negative and is dependent on the groups and geometry of the alkene. In the given alkenes the number of alkyl groups are different so their heat of hydrogenation will also be different. Also for same compound having two different geometrical isomers cis and trans, the hydrogenation energy will be different. For a given alkene more the alkyl groups or other substituents are attached, lesser will be the heat of hydrogenation. Lesser heat of hydrogenation means that the bonds are stronger and more stable, which means alkyl and other substitution on alkenes increases their stability. So, in the above compound (II) compound has the highest substitution of three alkyl groups and (I) has the lowest alkyl substitution of only one alkyl group whereas, compound (III)&(IV) both have two alkyl substitution. So now we can’t say about (III)&(IV) which one will have lower heat of hydrogenation. In case of cis and trans isomers, the trans isomers evolve less heat, this is because the cis isomers are more crowded due to non-bonded interactions between two alkyl groups on the same side of the double bond. So, compound (IV) is cis-butene whereas compound (III) is trans-butene, which means that compound (III) will have lower heat of hydrogenation. So, compound (II) will have the lowest heat of hydrogenation followed by compound (III), (IV) and (I).
So, the option will be (d).
Note:
Other than synthetic applications, catalytic hydrogenation is useful for analytical and thermochemical purposes. The amount of uptake of hydrogens by an unknown compound can give us an estimation about the unsaturation present in the system.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




