
Reaction mechanism of sulphonation of benzene ring using dil. $ HCl $ and $ {H_2}S{O_4} $ around $ {150^ \circ }C $ ?
Answer
493.5k+ views
Hint: Benzene is an aromatic rings that has high electron density due to the conjugation of double bonds in a cyclic manner inside the ring. Benzene ring tends to undergo nucleophilic substitution reactions that makes the removal of the sulfonic acid group possible.
Complete Step By Step Answer:
Benzene can easily undergo a sulphonation reaction in the presence of sulphuric acid. The sulfonic group can be removed by acidic hydrolysis.
Acids like dilute sulphuric acid or hydrochloric acid at high temperatures, allow the desulphonation reaction to take place. The water molecule acts as a Bronsted base and abstracts a proton from the acidic medium. Thy hydronium ion formed as a result of acification acts as an electrophile.
The electron rich double bond of benzene gets attacked by the electrophile in such a manner that the hydrogen abstracted from hydronium ions is placed on the carbon atom containing sulfonic acid group and a resonance stabilized carbocation is formed at the adjacent carbon position. Water is formed as a byproduct.
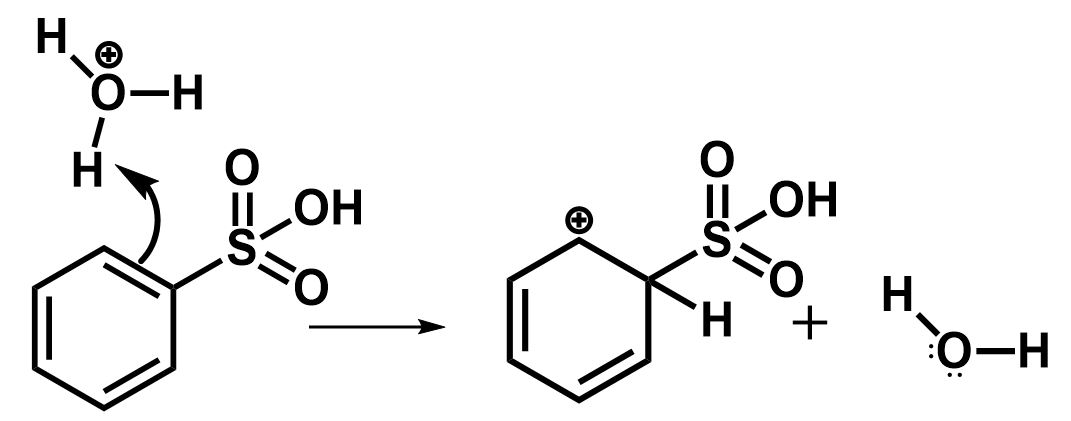
The next step involves the removal of the sulfonic group to regenerate the aromatic character. This is initiated by the nucleophilic attack of water molecules.
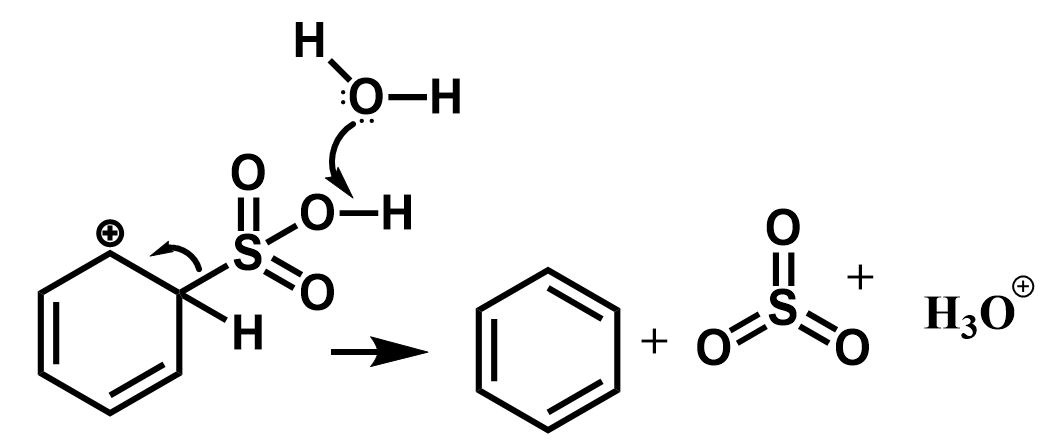
$ \Rightarrow $ As a result, the sulfonic acid group is removed in the form of sulphur trioxide and the benzene ring is regained.
Note:
The Sulphonation process is reversible in nature and therefore it is easy to remove the group. The attack of electrophile specifically shifts the electron density towards the sulfonic group containing carbon as it is an electron withdrawing group and a carbocation is more stable at the adjacent position.
Complete Step By Step Answer:
Benzene can easily undergo a sulphonation reaction in the presence of sulphuric acid. The sulfonic group can be removed by acidic hydrolysis.
Acids like dilute sulphuric acid or hydrochloric acid at high temperatures, allow the desulphonation reaction to take place. The water molecule acts as a Bronsted base and abstracts a proton from the acidic medium. Thy hydronium ion formed as a result of acification acts as an electrophile.
The electron rich double bond of benzene gets attacked by the electrophile in such a manner that the hydrogen abstracted from hydronium ions is placed on the carbon atom containing sulfonic acid group and a resonance stabilized carbocation is formed at the adjacent carbon position. Water is formed as a byproduct.

The next step involves the removal of the sulfonic group to regenerate the aromatic character. This is initiated by the nucleophilic attack of water molecules.

$ \Rightarrow $ As a result, the sulfonic acid group is removed in the form of sulphur trioxide and the benzene ring is regained.
Note:
The Sulphonation process is reversible in nature and therefore it is easy to remove the group. The attack of electrophile specifically shifts the electron density towards the sulfonic group containing carbon as it is an electron withdrawing group and a carbocation is more stable at the adjacent position.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




