
Reaction of t-butyl bromide with sodium methoxide produces:
(A) Isobutane
(B) Isobutylene
(C) Sodium- t- butoxide
(D) t- butyl methyl ether.
Answer
578.4k+ views
Hint: This method is called Williamson’s synthesis. General reaction of this synthesis is:
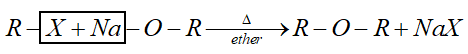
Complete step by step answer:
When alkyl halide is heated with alc. Sodium or potassium alkoxide gives corresponding ethers. Simple ethers can be easily prepared by this method.
But for preparation of mixed ether a proper choice of reactant is necessary. Primary alkyl halide is more susceptible to $S{N^2}$ reaction. Therefore, best yield of ether obtained when alkyl halide is primary and alkoxide is tertiary.
Example: tert-butyl ether is prepared by heating ethyl bromide with sodium tert-butoxide.
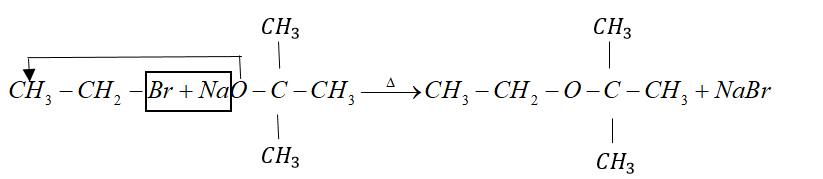
But when alkyl halide is secondary or tertiary the nucleophilic attack of alkoxide ion on $\alpha - $ carbon atom becomes difficult due to crowding effect.
Since alkoxide is a stronger base and attacking $\beta - $ hydrogen is easier. Therefore $\beta - $ elimination dominates.
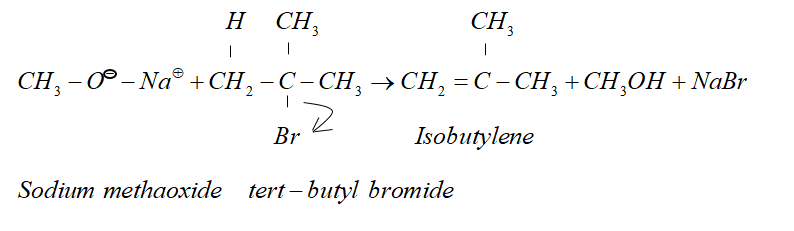
Therefore, in the given reaction isobutylene is formed.
So, the correct answer is “Option B”.
Note:
Williamson’s synthesis is a good method for preparation of mixed ether. But proper choice of reagent should be necessary. If we want to prepare t-butyl methyl ether then methyl bromide and sodium t-butoxide should be taken.
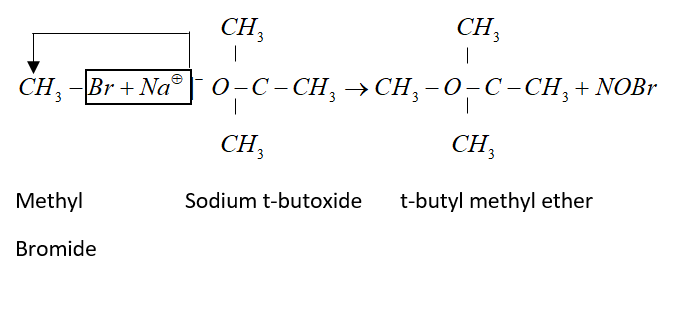
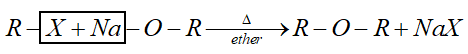
Complete step by step answer:
When alkyl halide is heated with alc. Sodium or potassium alkoxide gives corresponding ethers. Simple ethers can be easily prepared by this method.
But for preparation of mixed ether a proper choice of reactant is necessary. Primary alkyl halide is more susceptible to $S{N^2}$ reaction. Therefore, best yield of ether obtained when alkyl halide is primary and alkoxide is tertiary.
Example: tert-butyl ether is prepared by heating ethyl bromide with sodium tert-butoxide.
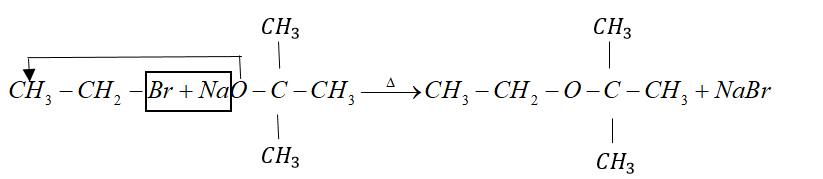
But when alkyl halide is secondary or tertiary the nucleophilic attack of alkoxide ion on $\alpha - $ carbon atom becomes difficult due to crowding effect.
Since alkoxide is a stronger base and attacking $\beta - $ hydrogen is easier. Therefore $\beta - $ elimination dominates.
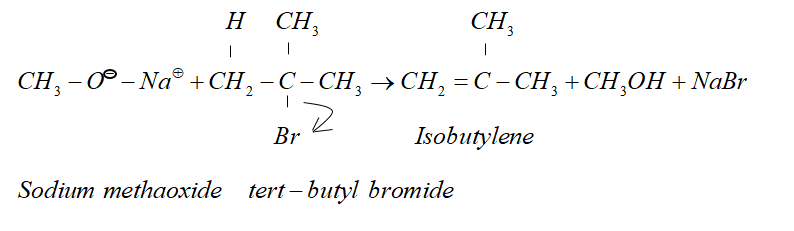
Therefore, in the given reaction isobutylene is formed.
So, the correct answer is “Option B”.
Note:
Williamson’s synthesis is a good method for preparation of mixed ether. But proper choice of reagent should be necessary. If we want to prepare t-butyl methyl ether then methyl bromide and sodium t-butoxide should be taken.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




