
What is the reason for these compounds to undergo $ {S_N}1 $ , $ {S_N}2 $ or both mechanisms?
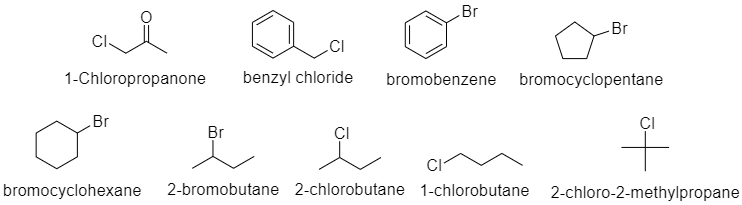
1-chloropropanone(chloroacetone), benzyl chloride, bromobenzene, bromocyclopentane, bromocyclohexane, 2-bromobutane, 2-chlorobutane, 1-chlorobutane, 2-chloro-2-methylpropane
Answer
528.6k+ views
Hint :In $ {S_N}2 $ reaction mechanism, the biggest barrier is steric hindrance. In $ {S_N}1 $ reactions, the barrier is the stability of the carbocation formed after the leaving group leaves. These factors along with the environmental conditions decide which mechanism the compound would undergo.
Complete Step By Step Answer:
We know that steric hindrance is the biggest barrier for $ {S_N}2 $ reactions, as the nucleophile attacks the backside of the compound. So due to their structure, the order of reactivity of the alkyl halide to undergo $ {S_N}2 $ mechanisms are $ 1^\circ > 2^\circ > > 3^\circ ({\text{worst)}} $ .
Also, for $ {S_N}1 $ reactions, the stability of the carbocation formed after the departure of the leaving group is the barrier. We are aware that tertiary carbocation is more stable than the secondary which is more stable than primary carbocation. So, the order of reactivity towards $ {S_N}1 $ mechanisms is $ 3^\circ > 2^\circ > > 1^\circ ({\text{worst)}} $ .
Here are the structures of the compounds given. Using the above rules, we can see that bromobenzene cannot undergo either of the reactions, because the carbocation formed is not stable and the nucleophile also will experience steric hindrance due the pi-bonds.
1-chloropropanone(chloroacetone), benzyl chloride and 1-chlorobutane will undergo $ {S_N}2 $ mechanism as they are primary alkyl-halides. Bromocyclopentane, bromocyclohexane and 2-bromobutane, 2-chlorobutane can undergo either of the mechanisms as they are secondary alkyl-halides. Moreover, it depends on the nucleophile and other conditions. 2-chloro-2-methylpropane can undergo $ {S_N}1 $ reaction, as it is a tertiary alkyl-halide.
Note :
Remember to look for the degree of the carbon atom to which the leaving group (halogen) is attached to determine the possibility of the compound to undergo either of the mechanisms. Also, remember that the nucleophile also plays a very important role in determining the type of reaction. $ {S_N}2 $ tends to proceed with strong nucleophiles, whereas $ {S_N}1 $ mechanism happens when the nucleophile is weak.
Complete Step By Step Answer:
We know that steric hindrance is the biggest barrier for $ {S_N}2 $ reactions, as the nucleophile attacks the backside of the compound. So due to their structure, the order of reactivity of the alkyl halide to undergo $ {S_N}2 $ mechanisms are $ 1^\circ > 2^\circ > > 3^\circ ({\text{worst)}} $ .
Also, for $ {S_N}1 $ reactions, the stability of the carbocation formed after the departure of the leaving group is the barrier. We are aware that tertiary carbocation is more stable than the secondary which is more stable than primary carbocation. So, the order of reactivity towards $ {S_N}1 $ mechanisms is $ 3^\circ > 2^\circ > > 1^\circ ({\text{worst)}} $ .
Here are the structures of the compounds given. Using the above rules, we can see that bromobenzene cannot undergo either of the reactions, because the carbocation formed is not stable and the nucleophile also will experience steric hindrance due the pi-bonds.
1-chloropropanone(chloroacetone), benzyl chloride and 1-chlorobutane will undergo $ {S_N}2 $ mechanism as they are primary alkyl-halides. Bromocyclopentane, bromocyclohexane and 2-bromobutane, 2-chlorobutane can undergo either of the mechanisms as they are secondary alkyl-halides. Moreover, it depends on the nucleophile and other conditions. 2-chloro-2-methylpropane can undergo $ {S_N}1 $ reaction, as it is a tertiary alkyl-halide.

Note :
Remember to look for the degree of the carbon atom to which the leaving group (halogen) is attached to determine the possibility of the compound to undergo either of the mechanisms. Also, remember that the nucleophile also plays a very important role in determining the type of reaction. $ {S_N}2 $ tends to proceed with strong nucleophiles, whereas $ {S_N}1 $ mechanism happens when the nucleophile is weak.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




