
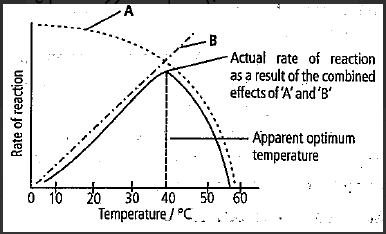
Refer to the given graph showing the relationship between temperature and enzyme action.
Select the correct statement regarding 'A' and 'B'.
(i) 'A' shows' rate at which reaction decreases due to denaturation of enzyme molecules.
(ii) 'B' shows the rate at which reaction increases due to decreased kinetic energy of the substrate.
(iii) As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall.
(iv) 'B' shows the rate at which reaction increases due to an increased kinetic energy of substrate and enzyme molecules.
A) i, iii, iv
B) iii only
C) iii and iv only
D) i and ii only

Answer
524.4k+ views
Hint: The relationship between the enzyme and temperature is that like most chemical reactions, the rate of enzyme-catalyzed reaction increases as the temperature is raised.
Complete answer:
Option A) i, iii, and iv. The graph below illustrates the relationship between temperature and enzyme activity. The rate at which the reaction slows down due to denaturation of enzyme molecules is shown in 'A.' As the temperature increases, more enzyme molecules are denatured, and the value of 'A' begins to decrease. 'B' shows the rate at which reaction increases due to increased kinetic energy of substrate and enzyme molecules.
Option B) iii only: As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall. This statement is true. Even though this statement is true there are other statements that are also true. iii only is not true.
Option C) iii and iv only: As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall. 'B' shows the rate at which reaction increases due to increased kinetic energy of substrate and enzyme molecules. B shows that the rate of reaction increases as the temperature reaches optimum value Option iii and iv are correct but there is another option that is also correct.
Option D) i and ii only: 'A' shows' rate at which reaction decreases due to denaturation of enzyme molecules. This sentence is true. 'B' shows the rate at which reaction increases due to decreased kinetic energy of the substrate. This is not correct. ‘B’ shows that the rate of reaction increases as the temperature reaches the optimum value. Sentence i is correct but sentence ii is not correct.
Hence the Correct answer is option A.
Note:
Proteins are denatured by heat. The given graph shows the relationship between temperature and enzyme action. The enzyme and substrate are treated with increasing temperature before they are mixed. Enzymes are mostly proteinaceous molecules. 'A' shows' rate at which reaction decreases due to denaturation of enzyme molecules. ‘B’ shows that the rate of reaction increases as the temperature reaches the optimum value. As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall. 'B' shows the rate at which reaction increases due to increased kinetic energy of substrate and enzyme molecules.
Complete answer:
Option A) i, iii, and iv. The graph below illustrates the relationship between temperature and enzyme activity. The rate at which the reaction slows down due to denaturation of enzyme molecules is shown in 'A.' As the temperature increases, more enzyme molecules are denatured, and the value of 'A' begins to decrease. 'B' shows the rate at which reaction increases due to increased kinetic energy of substrate and enzyme molecules.
Option B) iii only: As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall. This statement is true. Even though this statement is true there are other statements that are also true. iii only is not true.
Option C) iii and iv only: As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall. 'B' shows the rate at which reaction increases due to increased kinetic energy of substrate and enzyme molecules. B shows that the rate of reaction increases as the temperature reaches optimum value Option iii and iv are correct but there is another option that is also correct.
Option D) i and ii only: 'A' shows' rate at which reaction decreases due to denaturation of enzyme molecules. This sentence is true. 'B' shows the rate at which reaction increases due to decreased kinetic energy of the substrate. This is not correct. ‘B’ shows that the rate of reaction increases as the temperature reaches the optimum value. Sentence i is correct but sentence ii is not correct.
Hence the Correct answer is option A.
Note:
Proteins are denatured by heat. The given graph shows the relationship between temperature and enzyme action. The enzyme and substrate are treated with increasing temperature before they are mixed. Enzymes are mostly proteinaceous molecules. 'A' shows' rate at which reaction decreases due to denaturation of enzyme molecules. ‘B’ shows that the rate of reaction increases as the temperature reaches the optimum value. As temperature rises, more and more enzyme molecules are denatured and 'A' appears to fall. 'B' shows the rate at which reaction increases due to increased kinetic energy of substrate and enzyme molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




