
What is refining of metals? Explain with diagram the method of electrolysis by which copper is purified.
Answer
561k+ views
Hint: Refining of metals is a purification method used for making highest pure metals removing all the impurities. For this electrolysis method is employed which leads to around 100% of pure metal.
Complete step by step answer:
The refining of metals is a method used for obtaining ultrapure metals from pure metals. This method ensures the removal of most of the impurities by a process called electrolysis.
Electrolysis is process in which simultaneous oxidation and reduction reaction takes place in presence of electric current. Two electrodes are employed for electrolysis which is anode and cathode. At anode oxidation takes place and at cathode reduction reaction occurs.
All the metals are good conductors of electricity and their conducting power is utilized for their purification. The refining of copper metal is performed using the electrolysis method. The steps involved in this method are as follows:
The materials required for this process includes two copper rods and an electrolytic solution which is copper (II) sulfate. Out of the rods one is made of impure copper which serves as the anode and the rod made of pure copper acts as a cathode.
Both the rods are dipped into the solution of copper sulfate solution. The initiation of the process begins by passing an electric current through the electrolyte. The oxidation occurs at the anode and copper ions are released in the solution. The copper ion in the solution gets deposited at the cathode by the reduction process. Interestingly, the amount of copper released from anode and the amount deposited at the cathode are equal. The corresponding equations for the chemical reaction are
At anode (oxidation): \[Cu\left( s \right)\;\left( {Impure} \right)\; \to \;C{u^{2 + }}\left( {aq} \right) + 2{e^ - }\;\] At cathode (reduction): \[C{u^{2 + \;}}\left( {aq} \right) + 2{e^ - }\; \to \;Cu\left( s \right)\;\]
Thus, the deposited copper at the cathode electrode is nearly \[100\% \] pure.
The diagram of the method of electrolysis by which copper is purified is shown below:
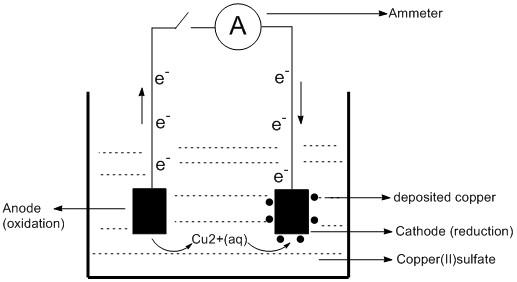
Note:
Copper exists in various ores as sulfides, sulphates or carbonates. The purification for each ore is different and is based on the impurities of the ore.
Complete step by step answer:
The refining of metals is a method used for obtaining ultrapure metals from pure metals. This method ensures the removal of most of the impurities by a process called electrolysis.
Electrolysis is process in which simultaneous oxidation and reduction reaction takes place in presence of electric current. Two electrodes are employed for electrolysis which is anode and cathode. At anode oxidation takes place and at cathode reduction reaction occurs.
All the metals are good conductors of electricity and their conducting power is utilized for their purification. The refining of copper metal is performed using the electrolysis method. The steps involved in this method are as follows:
The materials required for this process includes two copper rods and an electrolytic solution which is copper (II) sulfate. Out of the rods one is made of impure copper which serves as the anode and the rod made of pure copper acts as a cathode.
Both the rods are dipped into the solution of copper sulfate solution. The initiation of the process begins by passing an electric current through the electrolyte. The oxidation occurs at the anode and copper ions are released in the solution. The copper ion in the solution gets deposited at the cathode by the reduction process. Interestingly, the amount of copper released from anode and the amount deposited at the cathode are equal. The corresponding equations for the chemical reaction are
At anode (oxidation): \[Cu\left( s \right)\;\left( {Impure} \right)\; \to \;C{u^{2 + }}\left( {aq} \right) + 2{e^ - }\;\] At cathode (reduction): \[C{u^{2 + \;}}\left( {aq} \right) + 2{e^ - }\; \to \;Cu\left( s \right)\;\]
Thus, the deposited copper at the cathode electrode is nearly \[100\% \] pure.
The diagram of the method of electrolysis by which copper is purified is shown below:

Note:
Copper exists in various ores as sulfides, sulphates or carbonates. The purification for each ore is different and is based on the impurities of the ore.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




