
What is the relationship between pressure and volume?
Answer
478.5k+ views
Hint:To find the relationship between pressure and volume we see Boyle’s law. Boyle’s law states that at constant temperature the volume of the given gas is inversely proportional to the pressure of the given amount of gas.
Complete step by step answer:
Scientists studied the links between a gas's pressure $(P)$ , temperature $(T)$ , volume $(V)$ , and amount $(n)$ by maintaining two of the four variables constant(for example, amount temperature. Adjusting a third (for example, pressure), and evaluating the impact of the adjustment on the fourth (in this case, volume).
Boyle's Law gives us the relationship between volume and pressure.Since the gas particles are pressed closer together as the pressure on the gas increases, the volume of the gas decreases. When the pressure on a gas is decreased, the volume of the gas increases because the gas particles have now more space to move.
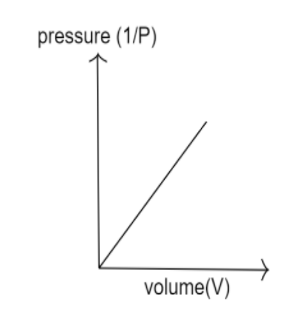
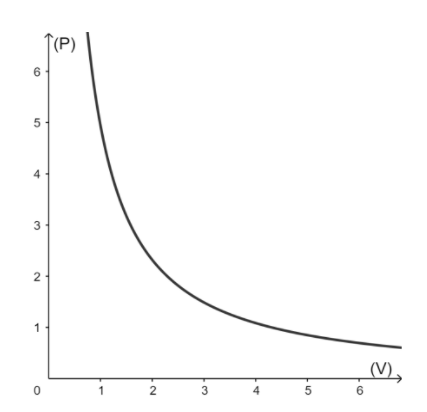
From the above graphs, we can see $V \propto \dfrac{1}{P}$. A plot of $V$ versus $\dfrac{1}{P}$ is thus a straight line having a constant slope. The constant's numerical value is determined by the amount of gas used in the experiment as well as the temperature at which it is conducted.
Note:The law can be deduced from the kinetic theory of gases under the assumption of an ideal (perfect) gas. At sufficiently low pressures, real gases satisfy Boyle's law, though the product $pv$ tends to decrease slightly at higher pressures when the gas begins to deviate from ideal behavior.
Complete step by step answer:
Scientists studied the links between a gas's pressure $(P)$ , temperature $(T)$ , volume $(V)$ , and amount $(n)$ by maintaining two of the four variables constant(for example, amount temperature. Adjusting a third (for example, pressure), and evaluating the impact of the adjustment on the fourth (in this case, volume).
Boyle's Law gives us the relationship between volume and pressure.Since the gas particles are pressed closer together as the pressure on the gas increases, the volume of the gas decreases. When the pressure on a gas is decreased, the volume of the gas increases because the gas particles have now more space to move.


From the above graphs, we can see $V \propto \dfrac{1}{P}$. A plot of $V$ versus $\dfrac{1}{P}$ is thus a straight line having a constant slope. The constant's numerical value is determined by the amount of gas used in the experiment as well as the temperature at which it is conducted.
Note:The law can be deduced from the kinetic theory of gases under the assumption of an ideal (perfect) gas. At sufficiently low pressures, real gases satisfy Boyle's law, though the product $pv$ tends to decrease slightly at higher pressures when the gas begins to deviate from ideal behavior.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




