
How can I represent an exothermic reaction in a potential energy diagram?
Answer
556.8k+ views
Hint: The potential energy diagram represents the change in the potential energy of the system when the reactant is converted to product. In an exothermic reaction, the heat is released by the system. The change in enthalpy is less than zero.
Complete step by step answer:
The exothermic reaction is defined as the reaction where the energy is released by the system. The enthalpy change of exothermic reaction is less than zero. $\Delta H < 0$.
The example for the exothermic reaction is the combustion of glucose.
The reaction is shown below.
${C_6}{H_{12}}{O_6} + 6{O_2} \to 6C{O_2} + 6{H_2}O$
In this reaction, one mole of glucose reacts with six moles of oxygen to form six mole of carbon dioxide and six mole of water.
During the chemical reaction, the energy change takes place which is shown by the diagram known as potential energy diagram.
The potential energy diagram shows the change in the potential energy of the system when the reactant compound changes to produce.
In the potential energy diagram, in y-axis potential energy is shown and in x-axis time is shown.
The potential energy diagram for the exothermic reaction is shown below.
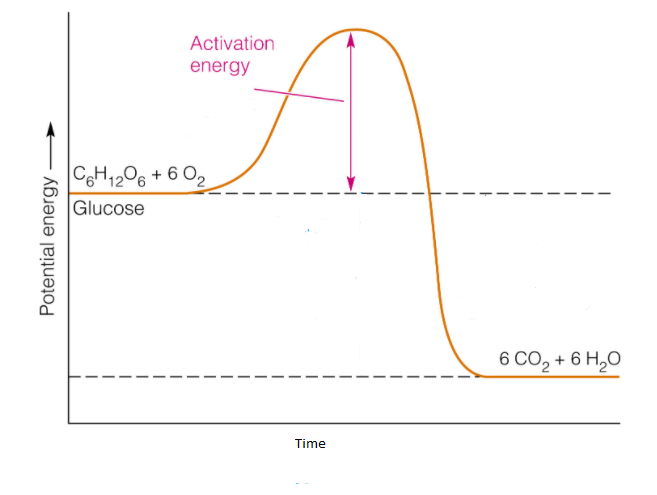
In the potential energy diagram, the potential energy of the reactant is higher than the potential energy of the product which can be seen in the diagram.
Note:
The difference in the potential energy of product and reactant is the enthalpy change.
$\Delta H = {H_f} - {H_I}$,
Where,
${H_f}$ is the enthalpy of product
${H_I}$ is the enthalpy of reactant
As, the ${H_f}$ is smaller than ${H_I}$, the enthalpy change $\Delta H$ is negative.
Complete step by step answer:
The exothermic reaction is defined as the reaction where the energy is released by the system. The enthalpy change of exothermic reaction is less than zero. $\Delta H < 0$.
The example for the exothermic reaction is the combustion of glucose.
The reaction is shown below.
${C_6}{H_{12}}{O_6} + 6{O_2} \to 6C{O_2} + 6{H_2}O$
In this reaction, one mole of glucose reacts with six moles of oxygen to form six mole of carbon dioxide and six mole of water.
During the chemical reaction, the energy change takes place which is shown by the diagram known as potential energy diagram.
The potential energy diagram shows the change in the potential energy of the system when the reactant compound changes to produce.
In the potential energy diagram, in y-axis potential energy is shown and in x-axis time is shown.
The potential energy diagram for the exothermic reaction is shown below.

In the potential energy diagram, the potential energy of the reactant is higher than the potential energy of the product which can be seen in the diagram.
Note:
The difference in the potential energy of product and reactant is the enthalpy change.
$\Delta H = {H_f} - {H_I}$,
Where,
${H_f}$ is the enthalpy of product
${H_I}$ is the enthalpy of reactant
As, the ${H_f}$ is smaller than ${H_I}$, the enthalpy change $\Delta H$ is negative.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




