
How many resonance structures are possible for naphthalene (Lack of formal charges)
A. 1
B. 2
C. 3
D. 4
Answer
516.3k+ views
Hint: The delocalization of the pi-electrons is the reason behind the formation of the resonance structures by the chemicals. The pi-bonds should be present in an alternate carbon to exhibit the resonance structures by a molecule.
Complete answer:
- In the question it is asked to find the number of resonance structures possible by a naphthalene molecule.
- First, we should know the structure of the naphthalene molecule to find the number of possible resonance structures.
- The structure of the naphthalene is as follows.
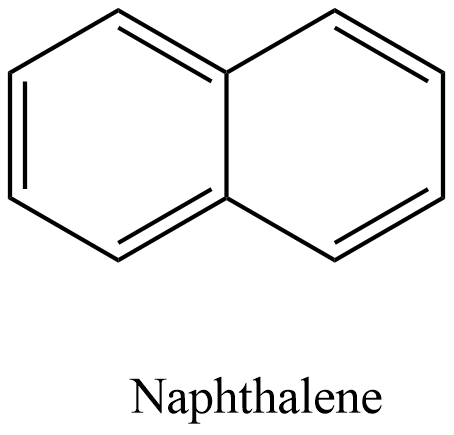
- There are two benzene rings present in the structure of the naphthalene molecule.
- We can see that there are five pi-bonds which are present in the structure of the naphthalene molecule.
- Those five pi-bonds are present in the alternate positions in the two rings.
- Now we can see the resonance structures of the pi-bonds in the below image.
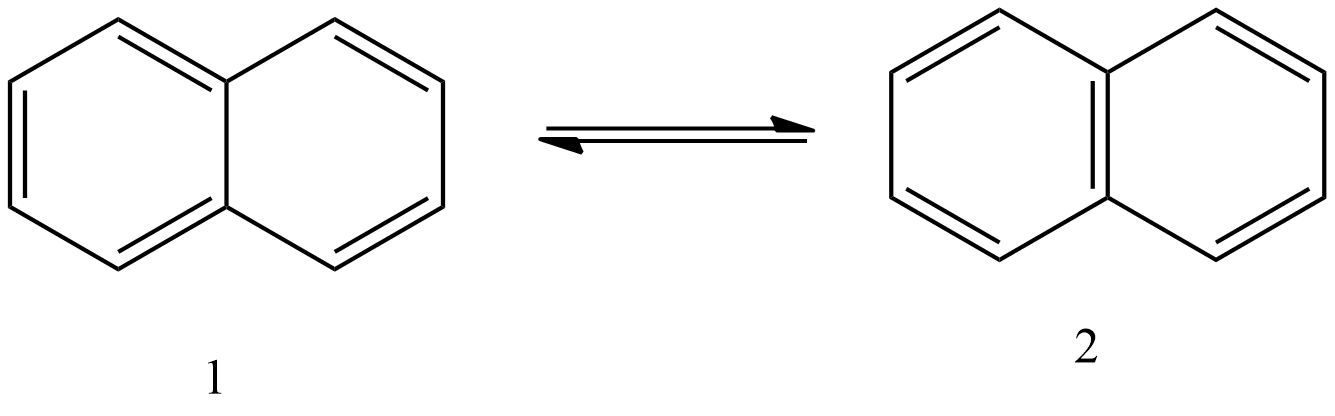
- In the above image we can see that the pi-bonds which are present in the benzene rings of the naphthalene molecule are going to delocalize between the carbon atoms and form two resonating structures.
- Therefore, the number of possible resonating structures by naphthalene are two.
So, the correct option is B.
Note:
All aromatic compounds which contain benzene rings in their structure are going to exhibit the resonance and show greater than the one structure for the respective organic compound due to the presence of the delocalized pi-electrons.
Complete answer:
- In the question it is asked to find the number of resonance structures possible by a naphthalene molecule.
- First, we should know the structure of the naphthalene molecule to find the number of possible resonance structures.
- The structure of the naphthalene is as follows.

- There are two benzene rings present in the structure of the naphthalene molecule.
- We can see that there are five pi-bonds which are present in the structure of the naphthalene molecule.
- Those five pi-bonds are present in the alternate positions in the two rings.
- Now we can see the resonance structures of the pi-bonds in the below image.

- In the above image we can see that the pi-bonds which are present in the benzene rings of the naphthalene molecule are going to delocalize between the carbon atoms and form two resonating structures.
- Therefore, the number of possible resonating structures by naphthalene are two.
So, the correct option is B.
Note:
All aromatic compounds which contain benzene rings in their structure are going to exhibit the resonance and show greater than the one structure for the respective organic compound due to the presence of the delocalized pi-electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




