
How many resonance structures can be drawn for ozone?
Answer
564.9k+ views
Hint: When a compound or molecule is going to represent two or more different hybrid structures and those structures have variation in the position of electrons then the structures are called resonance structures.
Complete answer:
- In the question it is asked to find how many resonance structures are possible for ozone.
- The molecular formula of ozone is ${{O}_{3}}$ means ozone contains three oxygen atoms in this molecular formula.
- In ozone the central oxygen atom is bonded to one oxygen atom with a single bond and to another oxygen atom the central oxygen atom is bonded with double bond.
- The net charge on the ozone molecule is zero.
- The possible two resonance structures of ozone molecules are as follows.
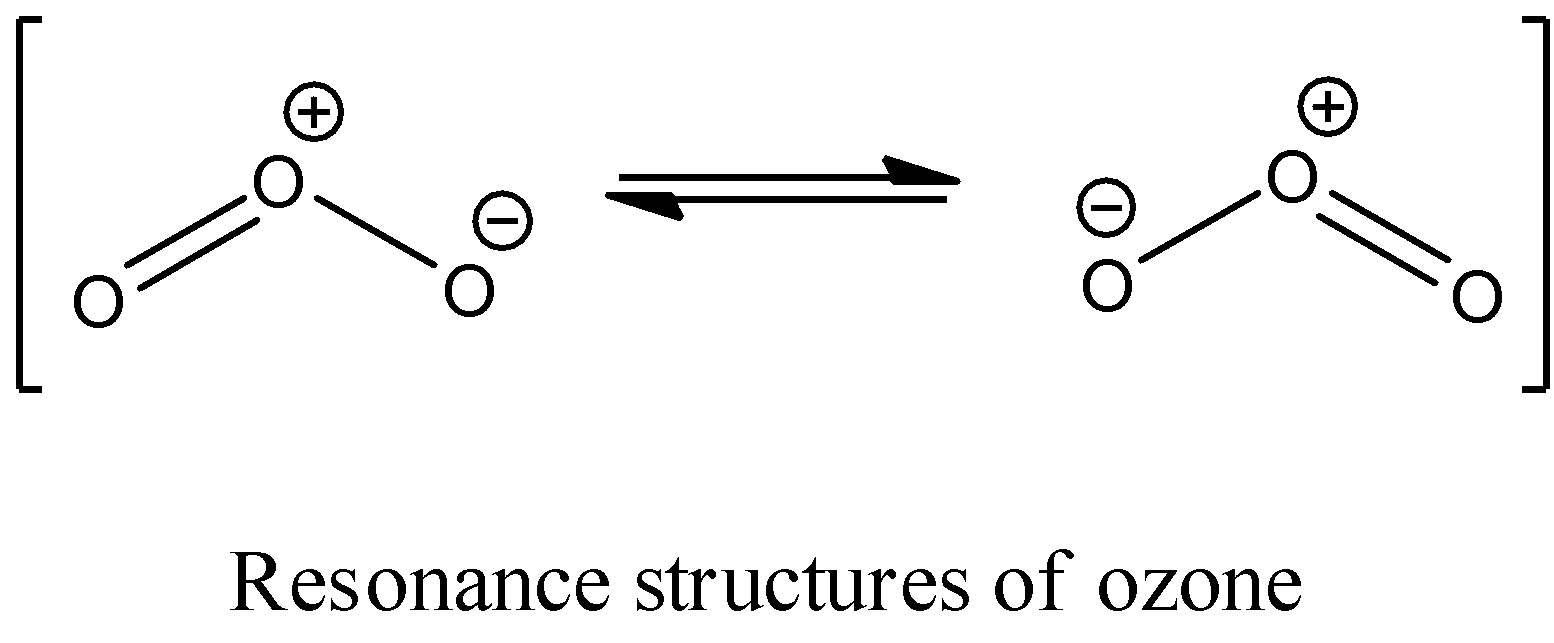
- In the above Lewis structures of ozone molecules the central oxygen atom carries ‘+1’ charge and the oxygen atom which is bonded with the central oxygen atom through a single bond has ‘-1‘charge.
- The two resonance structures possible for the ozone molecule have equivalent stability.
- Therefore only two resonance structures are possible for ozone molecules.
Note:
The overall charge between the resonance structures does not change. The charge will be going to change on individual atoms not on the total molecule. There are two oxygen atoms which are charged and one oxygen atom which has no charge on the oxygen atom in the ozone molecule. Ozone is a very light weight molecule and exists as gaseous in nature.
Complete answer:
- In the question it is asked to find how many resonance structures are possible for ozone.
- The molecular formula of ozone is ${{O}_{3}}$ means ozone contains three oxygen atoms in this molecular formula.
- In ozone the central oxygen atom is bonded to one oxygen atom with a single bond and to another oxygen atom the central oxygen atom is bonded with double bond.
- The net charge on the ozone molecule is zero.
- The possible two resonance structures of ozone molecules are as follows.

- In the above Lewis structures of ozone molecules the central oxygen atom carries ‘+1’ charge and the oxygen atom which is bonded with the central oxygen atom through a single bond has ‘-1‘charge.
- The two resonance structures possible for the ozone molecule have equivalent stability.
- Therefore only two resonance structures are possible for ozone molecules.
Note:
The overall charge between the resonance structures does not change. The charge will be going to change on individual atoms not on the total molecule. There are two oxygen atoms which are charged and one oxygen atom which has no charge on the oxygen atom in the ozone molecule. Ozone is a very light weight molecule and exists as gaseous in nature.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




