
How many resonance structures can be drawn for $\text{PO}_{\text{4}}^{\text{3}-}$-?
Answer
548.1k+ views
Hint: Resonance structures are sets of lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. They describe chemical bonding of a molecule or polyatomic ion.
Complete step by step answer:
When we draw resonance structures, we convert ion pairs to bonds and bonds to ione paris.
Resonance structures of any molecule can be drawn from the lewis structure of that molecule. Lewis structure of phosphate ion $\text{(PO}_{\text{4}}^{\text{3-}}\text{)}$
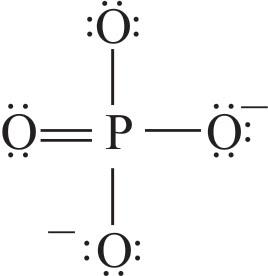
In Lewis structure of $\text{PO}_{\text{4}}^{\text{3}-}$ ion, there are three lone pairs in three oxygen atoms and other oxygen atom that is connected to phosphorus atom has two lone pairs and double bond.
Let's draw four stable resonance structures for the phosphate ion.
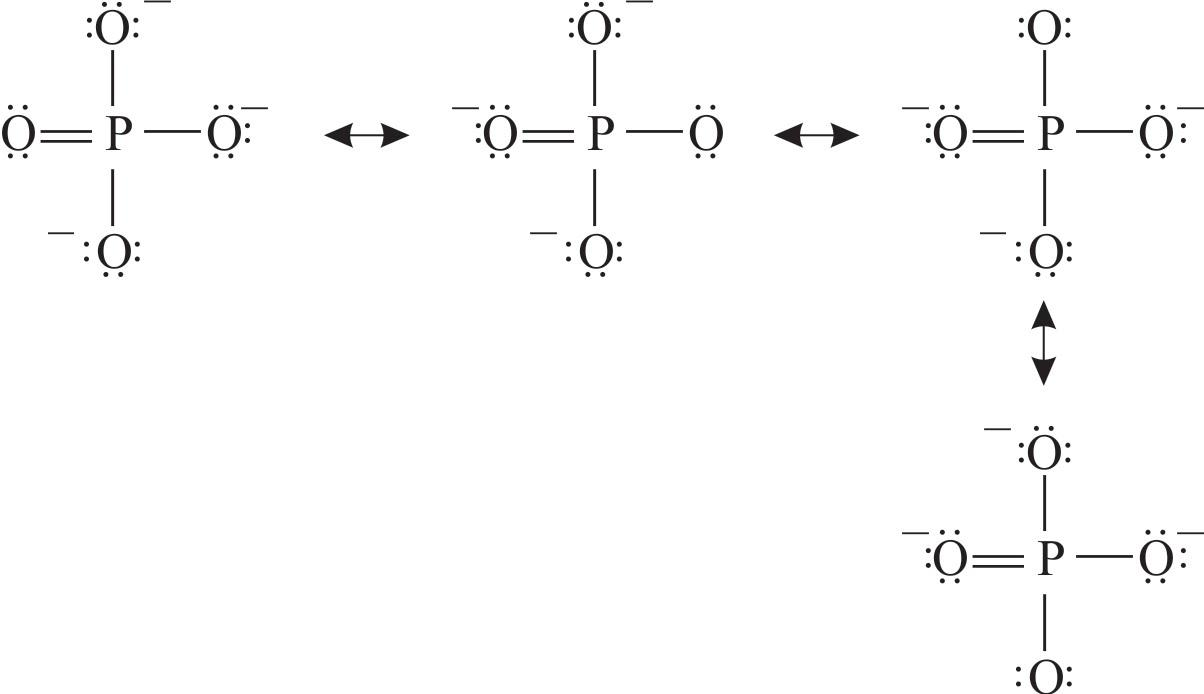
These are the four resonance structure of $\text{PO}_{4}^{3-}$
Note: From the above it is clear that, higher the resonance structure higher will be the stability of the molecule or ion. From various resonating structures, resonance hybrids will form. The contribution of resonating structure depends upon the stability of the resonating structure. The energy of resonating structures is higher than the energy of resonance hybrids. The resonance hybrid is the structure which is the most stable structure.
Complete step by step answer:
When we draw resonance structures, we convert ion pairs to bonds and bonds to ione paris.
Resonance structures of any molecule can be drawn from the lewis structure of that molecule. Lewis structure of phosphate ion $\text{(PO}_{\text{4}}^{\text{3-}}\text{)}$

In Lewis structure of $\text{PO}_{\text{4}}^{\text{3}-}$ ion, there are three lone pairs in three oxygen atoms and other oxygen atom that is connected to phosphorus atom has two lone pairs and double bond.
Let's draw four stable resonance structures for the phosphate ion.

These are the four resonance structure of $\text{PO}_{4}^{3-}$
Note: From the above it is clear that, higher the resonance structure higher will be the stability of the molecule or ion. From various resonating structures, resonance hybrids will form. The contribution of resonating structure depends upon the stability of the resonating structure. The energy of resonating structures is higher than the energy of resonance hybrids. The resonance hybrid is the structure which is the most stable structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




