
How many resonance structures does $C{H_3}{O^ - }$ have?
Answer
554.1k+ views
Hint: The methoxide ion $C{H_3}{O^ - }$ is an anion formed by gaining one electron where the negative charge is localized at the oxygen atom. In the structure no double bond is seen. When the structure is not explained by one Lewis structure, then the resonance effect is seen.
Complete step by step answer:
Lewis structure is defined as a representation of the valence electrons around the atoms of the molecule. The Lewis structure of the compound is used to show how the electrons are placed around the atoms of the compounds. The valence electrons are shown as small dots.
When any ion or compound is not explained by one Lewis structure, two or more sets of Lewis structure are formed and this is known as resonance effect.
The resonance effect is defined as the sets of Lewis structure which describes the delocalization of electrons in a molecule.
The methoxide is an organic anion which behaves as the conjugate base of methanol. The chemical formula of methoxide ion is $C{H_3}{O^ - }$.
The Lewis structure of methoxide ion is shown below.
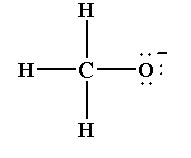
The negative charge of the anion is localized at the oxygen atom of the methoxide ion.
The structure of methoxide ion can be explained by one Lewis structure. So, no resonance structure is formed for methoxide ion $C{H_3}{O^ - }$.
Note:
The Lewis structure does not explain the geometry of the molecules, how the bonds are formed or how the valence electrons are shared between the atoms of the compound. The methoxide ion is a stronger base than hydroxide ions. It acts as a nucleophile.
Complete step by step answer:
Lewis structure is defined as a representation of the valence electrons around the atoms of the molecule. The Lewis structure of the compound is used to show how the electrons are placed around the atoms of the compounds. The valence electrons are shown as small dots.
When any ion or compound is not explained by one Lewis structure, two or more sets of Lewis structure are formed and this is known as resonance effect.
The resonance effect is defined as the sets of Lewis structure which describes the delocalization of electrons in a molecule.
The methoxide is an organic anion which behaves as the conjugate base of methanol. The chemical formula of methoxide ion is $C{H_3}{O^ - }$.
The Lewis structure of methoxide ion is shown below.

The negative charge of the anion is localized at the oxygen atom of the methoxide ion.
The structure of methoxide ion can be explained by one Lewis structure. So, no resonance structure is formed for methoxide ion $C{H_3}{O^ - }$.
Note:
The Lewis structure does not explain the geometry of the molecules, how the bonds are formed or how the valence electrons are shared between the atoms of the compound. The methoxide ion is a stronger base than hydroxide ions. It acts as a nucleophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




