
How many resonating structures are possible for the compound.

Answer
531.6k+ views
Hint: To solve this question, we first need to know what are resonating structures. Resonating structures or resonance structures can be used to depict the delocalization of electrons through a set of Lewis structures in an ion or a molecule containing multiple atoms.
Complete answer:
Sometimes, when a single Lewis structure cannot depict how atoms are bonded in an ion or a molecule due to the presence of fractional bonds or partial charges, the chemical bonding is depicted by resonance structure.
When the resonance structures of a molecule or an ion are merged to form a resonance hybrid.
A lone pair, when added to an atom, creates a negative charge on the molecule. Similarly, when a lone pair is removed from an atom, a positive charge is created on the atom.
When a bond is broken, a lone pair of electrons are added to the atom. Similarly, when a lone pair is added to an atom, a bond is formed.
Bonds and lone pairs delocalized in a molecule to form resonating structures. A resonance cycle is said to be complete when the original molecule is formed and the bonds and the lone pairs are the same as the original molecule.
It is important to note that while counting the resonating structures, the final structure which is the same as the original structure of the molecule should not be counted as one of the resonating structures.
The resonating structures of furan are as follows:
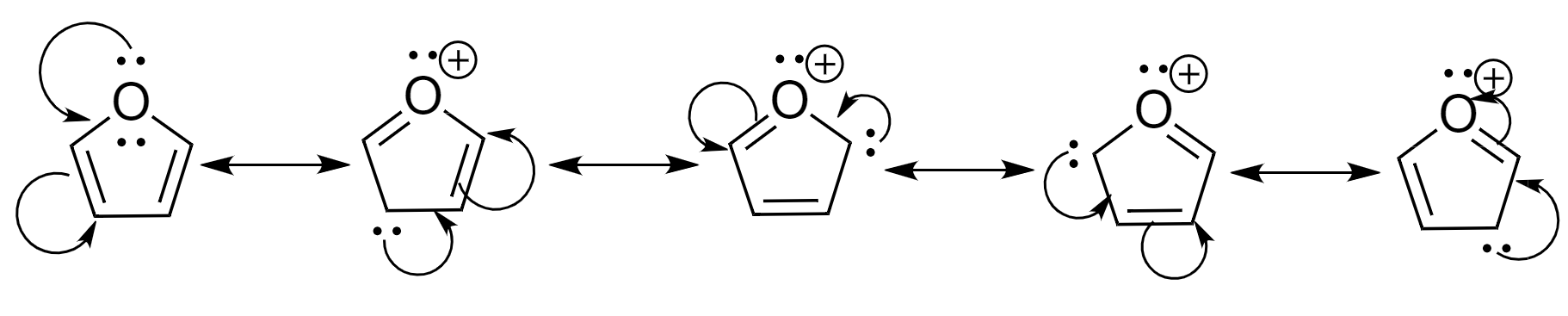
Hence, we can see that furan forms 5 resonating structures.
Note:
Some of the common mistakes that should be kept in mind while drawing the resonating structure of a molecule are
1. Atoms should not be moved around while drawing the resonating structure.
2. The charge and the number of lone pairs on the molecule must not change.
3. The curved arrows should be drawn correctly.
Complete answer:
Sometimes, when a single Lewis structure cannot depict how atoms are bonded in an ion or a molecule due to the presence of fractional bonds or partial charges, the chemical bonding is depicted by resonance structure.
When the resonance structures of a molecule or an ion are merged to form a resonance hybrid.
A lone pair, when added to an atom, creates a negative charge on the molecule. Similarly, when a lone pair is removed from an atom, a positive charge is created on the atom.
When a bond is broken, a lone pair of electrons are added to the atom. Similarly, when a lone pair is added to an atom, a bond is formed.
Bonds and lone pairs delocalized in a molecule to form resonating structures. A resonance cycle is said to be complete when the original molecule is formed and the bonds and the lone pairs are the same as the original molecule.
It is important to note that while counting the resonating structures, the final structure which is the same as the original structure of the molecule should not be counted as one of the resonating structures.
The resonating structures of furan are as follows:

Hence, we can see that furan forms 5 resonating structures.
Note:
Some of the common mistakes that should be kept in mind while drawing the resonating structure of a molecule are
1. Atoms should not be moved around while drawing the resonating structure.
2. The charge and the number of lone pairs on the molecule must not change.
3. The curved arrows should be drawn correctly.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




