
Rhombic sulphur consists of:
A.${S_8}$ chains
B.${S_2}$ molecules
C.${S_4}$ rings
D.${S_8}$ rings
Answer
584.4k+ views
Hint: Sulphur exists in several allotropic forms which may be classified into the following three categories:
-Homocyclic species containing six to twenty sulphur
-Catena sulphur having different chain polymers
-Unstable small molecules containing two to five sulphur atoms.
Complete step by step answer:
The two important allotropes of the homocyclic category are yellow rhombic and monoclinic sulphur. Rhombic sulphur is also known as α sulphur whereas monoclinic sulphur is known as β sulphur. These two forms are interconvertible and at the temperature above 369K rhombic sulphur gets converted to monoclinic and at the temp below 369K monoclinic sulphur changes to rhombic. Also the temperature of 369K both forms of sulphur exists and this temperature is called transition temperature.
Rhombic sulphur or orthorhombic sulphur are molecular solid which consists of ${S_8}$ a puckered ring having crown shape. It is also known as octahedral sulphur.
The ring structure of orthorhombic or α-sulphur is given below:
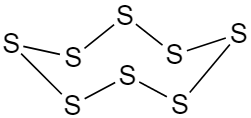
Hence the correct answer is option D.
Additional information: Rhombic sulphur is the most stable form of sulphur at room temperature and is prepared by slow evaporation of a solution of roll sulphur in carbon disulphide in the china dish. As a result, the octahedral crystal of rhombic sulphur appears. Rhombic sulphur has very small thermal and electrical conductivity and its melting point is 385.8 K.
Note:
Rhombic sulphur is insoluble in water but dissolves to some extent in benzene, alcohol, and ether and is easily soluble in carbon disulphide. When it dissolves in a boiling concentrated solution of sodium sulphite it forms sodium thiosulphate. The reaction for the same is given below:
$N{a_2}S{O_3} + S\xrightarrow{{Boil}}N{a_2}{S_2}{O_3}$
-Homocyclic species containing six to twenty sulphur
-Catena sulphur having different chain polymers
-Unstable small molecules containing two to five sulphur atoms.
Complete step by step answer:
The two important allotropes of the homocyclic category are yellow rhombic and monoclinic sulphur. Rhombic sulphur is also known as α sulphur whereas monoclinic sulphur is known as β sulphur. These two forms are interconvertible and at the temperature above 369K rhombic sulphur gets converted to monoclinic and at the temp below 369K monoclinic sulphur changes to rhombic. Also the temperature of 369K both forms of sulphur exists and this temperature is called transition temperature.
Rhombic sulphur or orthorhombic sulphur are molecular solid which consists of ${S_8}$ a puckered ring having crown shape. It is also known as octahedral sulphur.
The ring structure of orthorhombic or α-sulphur is given below:

Hence the correct answer is option D.
Additional information: Rhombic sulphur is the most stable form of sulphur at room temperature and is prepared by slow evaporation of a solution of roll sulphur in carbon disulphide in the china dish. As a result, the octahedral crystal of rhombic sulphur appears. Rhombic sulphur has very small thermal and electrical conductivity and its melting point is 385.8 K.
Note:
Rhombic sulphur is insoluble in water but dissolves to some extent in benzene, alcohol, and ether and is easily soluble in carbon disulphide. When it dissolves in a boiling concentrated solution of sodium sulphite it forms sodium thiosulphate. The reaction for the same is given below:
$N{a_2}S{O_3} + S\xrightarrow{{Boil}}N{a_2}{S_2}{O_3}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




