
Rutherford’s \[\alpha \] -particle scattering experiment led to the discovery of:
A. electrons
B. protons
C. neutrons
D. atomic nucleus
Answer
582.3k+ views
Hint: We know that, according to the model of an atom, the nucleus is positively charged due to the presence of protons in it and the negatively charged electrons revolve around it in a fixed circular orbit like planets spin around the sun.
Complete step by step solution:
We know that an atom is composed of three component particles which are electrons, protons and neutrons. The protons and neutrons are situated in the nucleus of an atom. However, the electrons are distributed uniformly outside the nucleus. There is no overall charge on an atom. This theory was proposed by Ernest Rutherford. Let’s discuss it in detail.
According to Rutherford, an atom is composed of a nucleus which is positively charged and the negatively charged electrons revolve around it in a fixed circular orbit. This conclusion was put forward on the basis of his scattering experiment.
The experiment performed by Rutherford gave important observations about an atom.
1. The first observations of the alpha particle scattering experiment explains that most of the alpha particles passed straight from the foil without any failure in their direction. This observation confirms that there must be sufficient free space within the atom.
2. The second observation states that most of the alpha particles undergo deflection. Some particles experience small deflection whereas some particles experience large deflection. This observation confirms that there must be a positively charged body in an atom due to which the positively charged alpha particles experience repulsive force which lead to the small and large deflection. This confirms the presence of a heavy positively charged nucleus in the center of the atom.
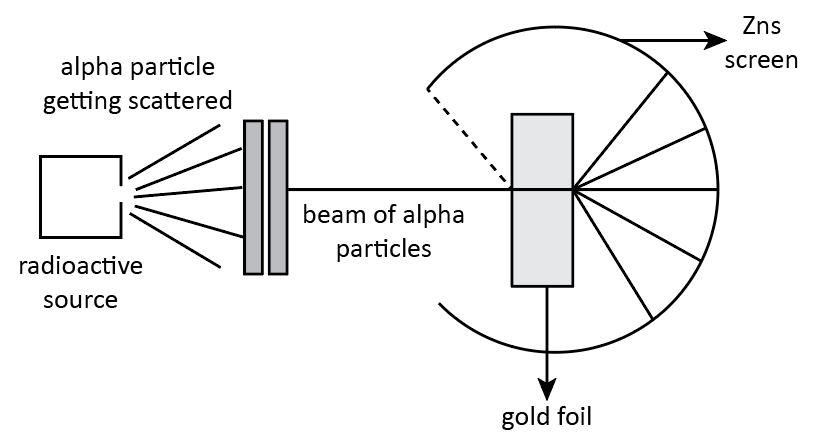
Hence, we can say that Rutherford's \[\alpha \] -particle scattering experiment led to the discovery of atomic nuclei.
Thus, the correct option is D.
Note:
As we know that, the mass and properties of all the atoms present in an element are identical. After the discovery of atoms, the different subatomic particles were also discovered by various experiments done by scientists. In the year, $1911$, Ernest Rutherford describes the structure of an atom. This model is called the planetary model of the atom.
Complete step by step solution:
We know that an atom is composed of three component particles which are electrons, protons and neutrons. The protons and neutrons are situated in the nucleus of an atom. However, the electrons are distributed uniformly outside the nucleus. There is no overall charge on an atom. This theory was proposed by Ernest Rutherford. Let’s discuss it in detail.
According to Rutherford, an atom is composed of a nucleus which is positively charged and the negatively charged electrons revolve around it in a fixed circular orbit. This conclusion was put forward on the basis of his scattering experiment.
The experiment performed by Rutherford gave important observations about an atom.
1. The first observations of the alpha particle scattering experiment explains that most of the alpha particles passed straight from the foil without any failure in their direction. This observation confirms that there must be sufficient free space within the atom.
2. The second observation states that most of the alpha particles undergo deflection. Some particles experience small deflection whereas some particles experience large deflection. This observation confirms that there must be a positively charged body in an atom due to which the positively charged alpha particles experience repulsive force which lead to the small and large deflection. This confirms the presence of a heavy positively charged nucleus in the center of the atom.

Hence, we can say that Rutherford's \[\alpha \] -particle scattering experiment led to the discovery of atomic nuclei.
Thus, the correct option is D.
Note:
As we know that, the mass and properties of all the atoms present in an element are identical. After the discovery of atoms, the different subatomic particles were also discovered by various experiments done by scientists. In the year, $1911$, Ernest Rutherford describes the structure of an atom. This model is called the planetary model of the atom.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




