
How many secondary carbon atoms are there in ${{\left( C{{H}_{3}} \right)}_{2}}CHC{{H}_{2}}CH{{\left( C{{H}_{3}} \right)}_{2}}$?
Answer
517.5k+ views
Hint: A hydrocarbon chain may involve various carbon atoms. These are termed according to the carbon atoms attached adjacent to them. When a carbon has only one carbon atom attached, it is termed as primary carbon atom, while when it has 2 carbon atoms attached to it is called as secondary carbon atom. To recognize them a structural formula has to be drawn.
Complete answer:
A hydrocarbon long chain may have alkyl groups attached at various positions that define the nature of the attached carbon atoms as primary $1{}^\circ $, secondary $2{}^\circ $and tertiary $3{}^\circ $. There are hydrogen atoms attached with these carbon atoms that are also termed in the same way as primary, secondary, and tertiary.
We have been given a compound that has a formula ${{\left( C{{H}_{3}} \right)}_{2}}CHC{{H}_{2}}CH{{\left( C{{H}_{3}} \right)}_{2}}$, we have to identify the secondary carbon atoms. For this let’s see the structural formula and label the carbon atoms as, 1 to 5.
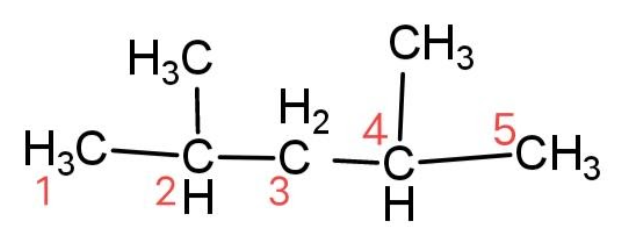
The secondary carbon atom is the carbon atom that is attached with two other adjacent carbon atoms. So the secondary carbon atom is carbon 3.
Hence, there is only 1 secondary carbon atom present in ${{\left( C{{H}_{3}} \right)}_{2}}CHC{{H}_{2}}CH{{\left( C{{H}_{3}} \right)}_{2}}$.
Note:
The compound is called 2, 4 – dimethyl pentane. In this given compound there are 2 primary carbon atoms that are 1 and 5 carbons that are attached with only one carbon atom. Similarly there are 2 tertiary carbon atoms that are carbon 2 and carbon 4 that have 2 carbon atoms attached to them.
Complete answer:
A hydrocarbon long chain may have alkyl groups attached at various positions that define the nature of the attached carbon atoms as primary $1{}^\circ $, secondary $2{}^\circ $and tertiary $3{}^\circ $. There are hydrogen atoms attached with these carbon atoms that are also termed in the same way as primary, secondary, and tertiary.
We have been given a compound that has a formula ${{\left( C{{H}_{3}} \right)}_{2}}CHC{{H}_{2}}CH{{\left( C{{H}_{3}} \right)}_{2}}$, we have to identify the secondary carbon atoms. For this let’s see the structural formula and label the carbon atoms as, 1 to 5.

The secondary carbon atom is the carbon atom that is attached with two other adjacent carbon atoms. So the secondary carbon atom is carbon 3.
Hence, there is only 1 secondary carbon atom present in ${{\left( C{{H}_{3}} \right)}_{2}}CHC{{H}_{2}}CH{{\left( C{{H}_{3}} \right)}_{2}}$.
Note:
The compound is called 2, 4 – dimethyl pentane. In this given compound there are 2 primary carbon atoms that are 1 and 5 carbons that are attached with only one carbon atom. Similarly there are 2 tertiary carbon atoms that are carbon 2 and carbon 4 that have 2 carbon atoms attached to them.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




