
Select the correct statement for ${P_4}{O_{10}}$.
(This question has multiple correct options)
A.It has four $s{p^3}$ hybridised phosphorus atoms
B.It has high $s\% $ character in P-O bond than the ${P_4}{O_6}$
C.It has a cage like structure
D.It has ${p_\pi } - {d_\pi }$ bonding
Answer
560.7k+ views
Hint: ${P_4}{O_{10}}$ is an oxide of phosphorus. Its chemical name is phosphorus pentoxide, because it is a dimer of ${P_2}{O_5}$ . In ${P_4}{O_{10}}$ , oxygen is in $ - 2$ oxidation state and phosphorus is in $ + 5$ oxidation state.
Complete step by step answer:
Structure of ${P_4}{O_{10}}$ is shown below.
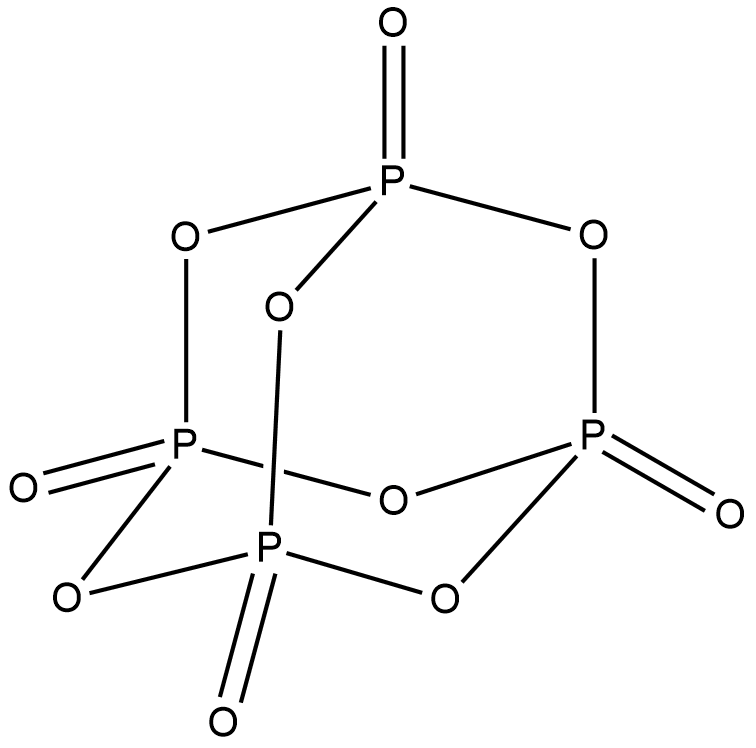
In ${P_4}{O_{10}}$ , all the phosphorus atoms are $s{p^3}$ hybridised with a tetrahedral structure. $s{p^3}d$ hybridisation is preferred for phosphorus atom over $s{p^3}$ hybridisation. But for ${P_4}{O_{10}}$ , $s{p^3}$ hybridisation is more stable than $s{p^3}d$. Hence ${P_4}{O_{10}}$has four $s{p^3}$ hybridised phosphorus atoms. Option A is correct.
${P_4}{O_6}$ is another oxide of phosphorus. Both ${P_4}{O_6}$ and ${P_4}{O_{10}}$ contains six P-O-P bond and $4$ six membered ring. In ${P_4}{O_6}$ , the atomic orbitals containing lone pairs have more s-character and less p-character. Hence they have shorter bond length. But the bonding orbitals have more p-character and less s-character and hence a longer bond length. But in ${P_4}{O_{10}}$ the bonding orbitals have more $s\% $ character compared to that in ${P_4}{O_6}$ . The bonding orbitals means the P-O bond. Hence ${P_4}{O_6}$ has a higher $s\% $ character in P-O bond than the ${P_4}{O_6}$. Option B is also correct.
From the structure given above, we can see that ${P_4}{O_{10}}$ has a cage like structure. Hence option C is correct.
The terminal P-O bonds in ${P_4}{O_{10}}$ are formed by ${p_\pi } - {d_\pi }$ bonding. p-orbitals of oxygen overlap with empty d-orbitals of phosphorus. The phosphorus atom donates its lone pair to this bonding forming a $\pi $ - back bonding. Hence option D is correct.
Options A,B,C and D correct for ${P_4}{O_{10}}$ .
Note:
Phosphorus can show $ + 3$ and $ + 5$ oxidation states. Hence it forms two oxides ${P_2}{O_3}$ and ${P_2}{O_5}$ with oxidation states $ + 3$ and $ + 5$ respectively. But their monomeric form is not stable. This is the reason why they exist as dimer - ${P_4}{O_6}$ and ${P_4}{O_{10}}$.
Complete step by step answer:
Structure of ${P_4}{O_{10}}$ is shown below.

In ${P_4}{O_{10}}$ , all the phosphorus atoms are $s{p^3}$ hybridised with a tetrahedral structure. $s{p^3}d$ hybridisation is preferred for phosphorus atom over $s{p^3}$ hybridisation. But for ${P_4}{O_{10}}$ , $s{p^3}$ hybridisation is more stable than $s{p^3}d$. Hence ${P_4}{O_{10}}$has four $s{p^3}$ hybridised phosphorus atoms. Option A is correct.
${P_4}{O_6}$ is another oxide of phosphorus. Both ${P_4}{O_6}$ and ${P_4}{O_{10}}$ contains six P-O-P bond and $4$ six membered ring. In ${P_4}{O_6}$ , the atomic orbitals containing lone pairs have more s-character and less p-character. Hence they have shorter bond length. But the bonding orbitals have more p-character and less s-character and hence a longer bond length. But in ${P_4}{O_{10}}$ the bonding orbitals have more $s\% $ character compared to that in ${P_4}{O_6}$ . The bonding orbitals means the P-O bond. Hence ${P_4}{O_6}$ has a higher $s\% $ character in P-O bond than the ${P_4}{O_6}$. Option B is also correct.
From the structure given above, we can see that ${P_4}{O_{10}}$ has a cage like structure. Hence option C is correct.
The terminal P-O bonds in ${P_4}{O_{10}}$ are formed by ${p_\pi } - {d_\pi }$ bonding. p-orbitals of oxygen overlap with empty d-orbitals of phosphorus. The phosphorus atom donates its lone pair to this bonding forming a $\pi $ - back bonding. Hence option D is correct.
Options A,B,C and D correct for ${P_4}{O_{10}}$ .
Note:
Phosphorus can show $ + 3$ and $ + 5$ oxidation states. Hence it forms two oxides ${P_2}{O_3}$ and ${P_2}{O_5}$ with oxidation states $ + 3$ and $ + 5$ respectively. But their monomeric form is not stable. This is the reason why they exist as dimer - ${P_4}{O_6}$ and ${P_4}{O_{10}}$.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




