
Select the correct statement(s).
A. In diborane 12 valence ${e}^{-}$ are involved in bonding
B. In diborane, maximum six atoms, two boron and four terminal hydrogen, lie in the same plane.
C. Diborane has ethane-like structure.
D. In diborane, bridging bonds are stronger and longer than the terminal bonds.
Answer
596.4k+ views
Hint: Diborane is a chemical compound which consists of two elements, boron and hydrogen. The chemical formula of diborane is ${B}_{2}{H}_{6}$. It is used as a reducing agent and is used as a catalyst for hydrocarbon polymerization.
Complete step by step solution:
Diborane is a colorless, pyrophoric gas. It has a repulsively sweet odor. Let us now look at the options one by one.
(A). In diborane 12 valence electrons are involved in bonding: Let us first look at the structure of diborane.
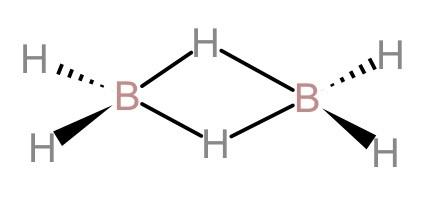
Now, by looking at the structure of diborane, we can see that there are 12 valence electrons that are involved in bonding. 8 valence electrons are used in the four non-bridging hydrogen bonds and four valence electrons are shared in forming 2-centered 2-electron bonds. Therefore, this statement is correct.
(B). In diborane, maximum six atoms, two boron and four terminal hydrogen, lie in the same plane: By looking at the structure of diborane, we can see that two boron atoms and the four terminal hydrogen atoms of the molecule are in the same plane. And the bridging hydrogen atoms lie above and below this plane. Therefore, this statement is also correct.
(C). Diborane has ethane-like structure: The structure of ethane has a single bond between the two carbon atoms but in diborane, there are bridged hydrogen-boron bonds. Therefore, it does not have an ethane-like structure. Hence, this is not correct.
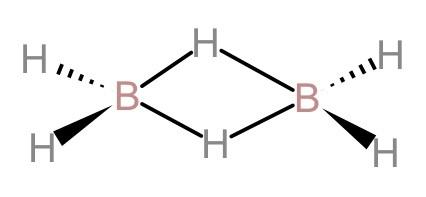
(D). In diborane, bridging bonds are stronger and longer than the terminal bonds: The bridging hydrogen bonds in diborane are stronger, which means that the bridging H-atoms cannot be easily replaced in a chemical reaction. The length of the bridging hydrogen-boron bonds is $1.33 \overset {0}{A}$ which is longer than the length of the terminal hydrogen-boron length which is $1.19 \overset {0}{A}$. And we know that, the longer the bond, the stronger the bond. Therefore, the bridging bonds are stronger and longer than the terminal bonds. Hence, this statement is also correct.
Therefore, the correct statements are (A), (B) and (D).
Note: Diborane is known to be an electron deficient compound. It is highly reactive and a versatile reagent. It is also used as a rocket propellant. The complete combustion of diborane is strongly exothermic.
Complete step by step solution:
Diborane is a colorless, pyrophoric gas. It has a repulsively sweet odor. Let us now look at the options one by one.
(A). In diborane 12 valence electrons are involved in bonding: Let us first look at the structure of diborane.

Now, by looking at the structure of diborane, we can see that there are 12 valence electrons that are involved in bonding. 8 valence electrons are used in the four non-bridging hydrogen bonds and four valence electrons are shared in forming 2-centered 2-electron bonds. Therefore, this statement is correct.
(B). In diborane, maximum six atoms, two boron and four terminal hydrogen, lie in the same plane: By looking at the structure of diborane, we can see that two boron atoms and the four terminal hydrogen atoms of the molecule are in the same plane. And the bridging hydrogen atoms lie above and below this plane. Therefore, this statement is also correct.
(C). Diborane has ethane-like structure: The structure of ethane has a single bond between the two carbon atoms but in diborane, there are bridged hydrogen-boron bonds. Therefore, it does not have an ethane-like structure. Hence, this is not correct.

(D). In diborane, bridging bonds are stronger and longer than the terminal bonds: The bridging hydrogen bonds in diborane are stronger, which means that the bridging H-atoms cannot be easily replaced in a chemical reaction. The length of the bridging hydrogen-boron bonds is $1.33 \overset {0}{A}$ which is longer than the length of the terminal hydrogen-boron length which is $1.19 \overset {0}{A}$. And we know that, the longer the bond, the stronger the bond. Therefore, the bridging bonds are stronger and longer than the terminal bonds. Hence, this statement is also correct.
Therefore, the correct statements are (A), (B) and (D).
Note: Diborane is known to be an electron deficient compound. It is highly reactive and a versatile reagent. It is also used as a rocket propellant. The complete combustion of diborane is strongly exothermic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




