
Select the correct statement(s) about the compound $ NO\left[{\rm BF}_4\right] $
(A) It has $ 5\ \sigma $ and $ 2 \pi$ bonds
(B) Nitrogen-oxygen bond length is higher than nitric oxide (NO)
(C) It is a diamagnetic species
(D) BF bond length in this compound is lower than in $ BF_3 $
Answer
510k+ views
Hint: Using the structure of $ NO\left[{\rm BF}_4\right]\ $ and its bonding elements, this question can be answered. Sigma bonds are formed head-on between orbitals while pi bonds are formed laterally between two orbitals. The Lewis structure of the compound can be used.
Complete answer:
First, the structure of $ NO\left[{\rm BF}_4\right] $
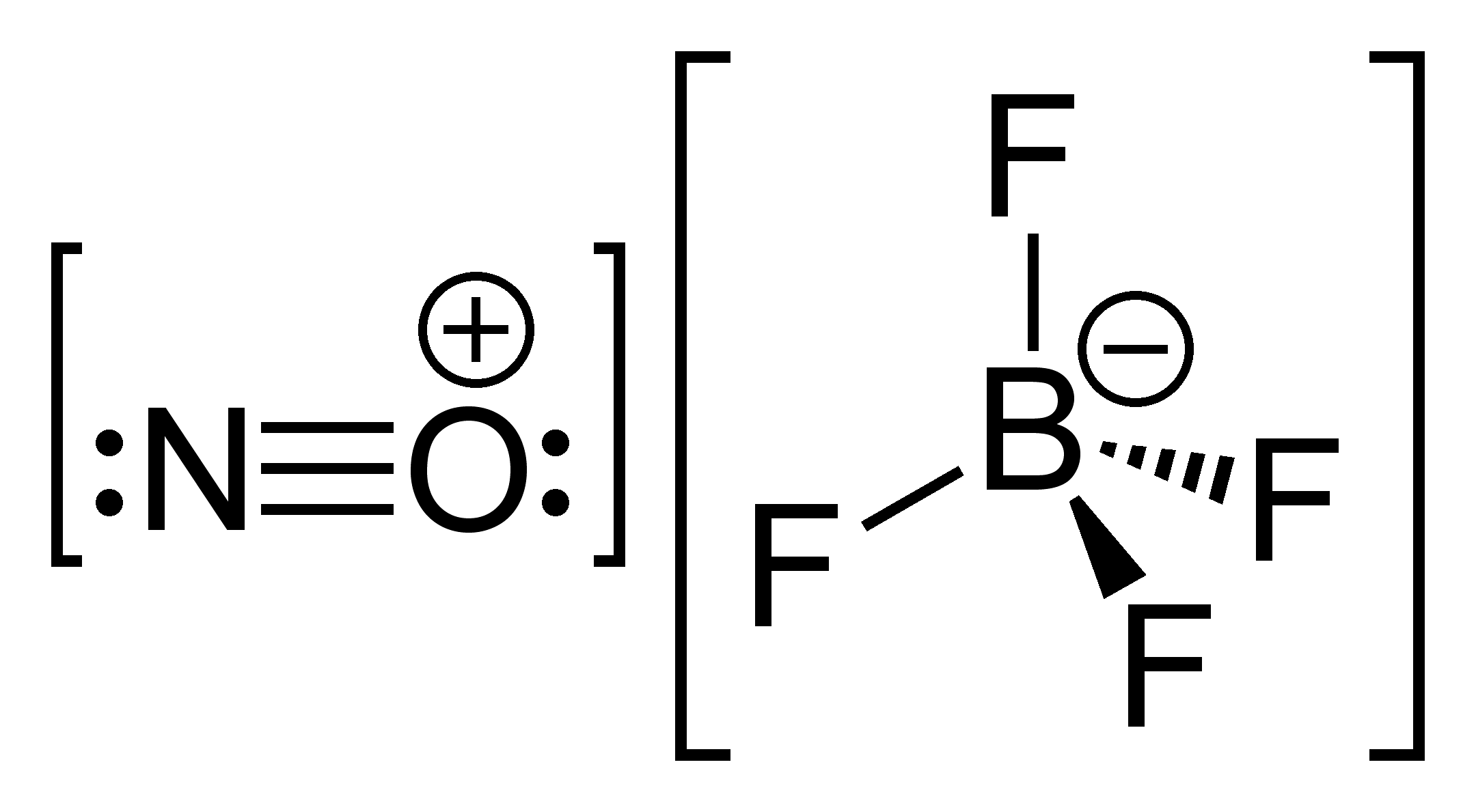
Bond Order (B.O.) is the number of chemical bonds between a pair of atoms and it indicates the stability of the bond too.
The number of bonds in $ \left[BF_4\right]^-= 4 $
B.O. of $NO^+$ = $ 3.0 $ (it has one $\sigma$ and two $\pi$ bonds making a triple bond as seen in the structure)
Therefore, the total number of $ \sigma $ bonds = $ 5 $ ; and total number of $\pi$ bonds = $ 2 $
Also, B.O. of $NO^+$ is $ 3.0 $ , while the B.O. of NO is $ 2.5 $
The higher the bond order, the stronger the bond is which implies the bond length is shorter.
Therefore, bond length of $ NO^+ $ is shorter than that of nitric oxide (NO).
Diamagnetism depends on whether there are only paired electrons. $ NO^+ $ and $ \left[BF_4\right]^- $ Both are diamagnetic.
Finally, since there is no p-p back bonding in $ \left[BF_4\right]^-$ the B-F bonds are longer in $ \left[BF_4\right]^- $ compared to $ BF_3 $ .
Therefore, with all these points, options (A) and (C) are the correct ones for this question.
Note:
Such questions require a good understanding of structures and bonding elements. Also, terms such as bond order, bond length, paired and unpaired electrons and diamagnetism need to be known along with their relationship with each other.
Complete answer:
First, the structure of $ NO\left[{\rm BF}_4\right] $
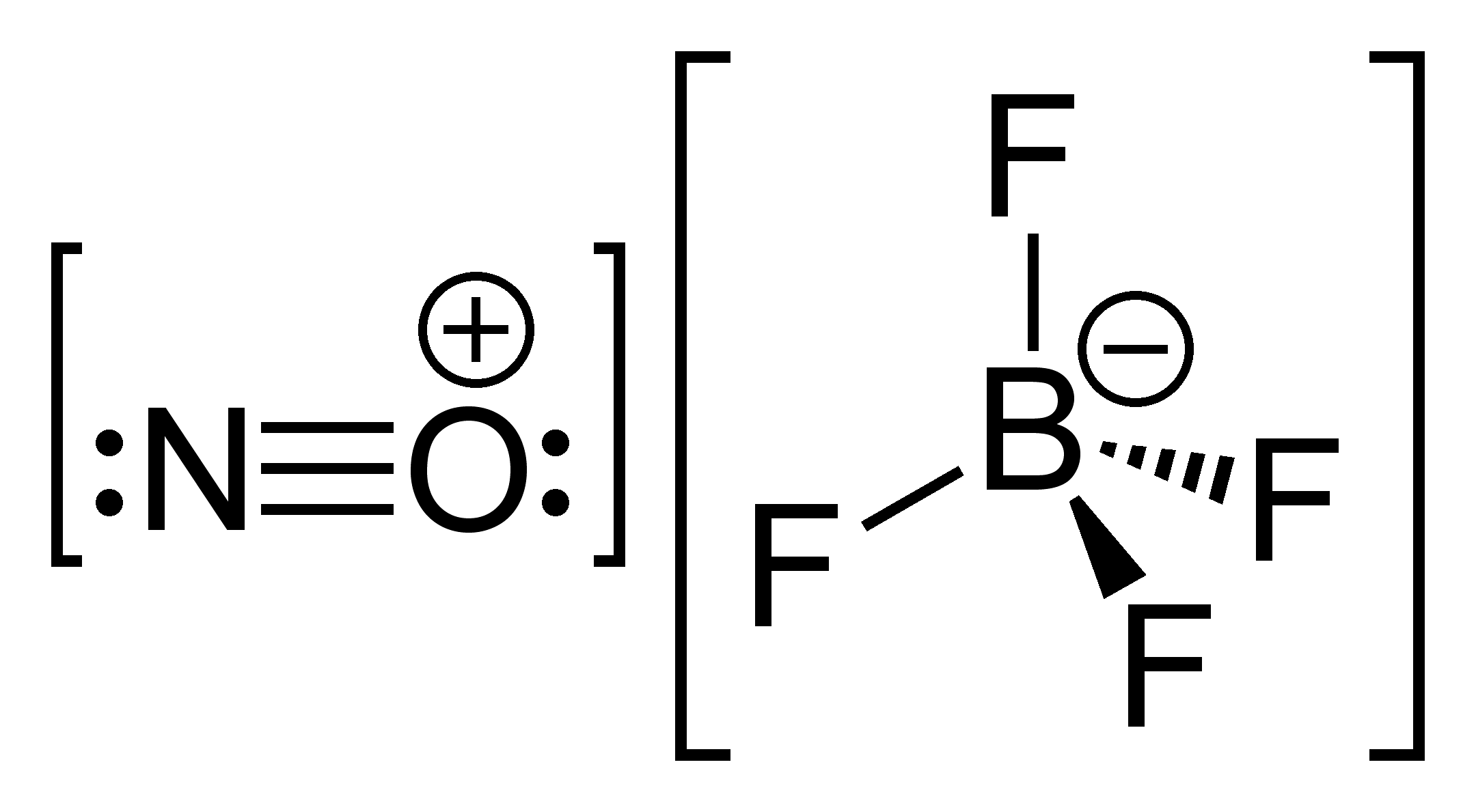
Bond Order (B.O.) is the number of chemical bonds between a pair of atoms and it indicates the stability of the bond too.
The number of bonds in $ \left[BF_4\right]^-= 4 $
B.O. of $NO^+$ = $ 3.0 $ (it has one $\sigma$ and two $\pi$ bonds making a triple bond as seen in the structure)
Therefore, the total number of $ \sigma $ bonds = $ 5 $ ; and total number of $\pi$ bonds = $ 2 $
Also, B.O. of $NO^+$ is $ 3.0 $ , while the B.O. of NO is $ 2.5 $
The higher the bond order, the stronger the bond is which implies the bond length is shorter.
Therefore, bond length of $ NO^+ $ is shorter than that of nitric oxide (NO).
Diamagnetism depends on whether there are only paired electrons. $ NO^+ $ and $ \left[BF_4\right]^- $ Both are diamagnetic.
Finally, since there is no p-p back bonding in $ \left[BF_4\right]^-$ the B-F bonds are longer in $ \left[BF_4\right]^- $ compared to $ BF_3 $ .
Therefore, with all these points, options (A) and (C) are the correct ones for this question.
Note:
Such questions require a good understanding of structures and bonding elements. Also, terms such as bond order, bond length, paired and unpaired electrons and diamagnetism need to be known along with their relationship with each other.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




