
Select the structure of the major product formed when 1-methylcyclobutane reacts with $ B{r_2} $ in presence of light.
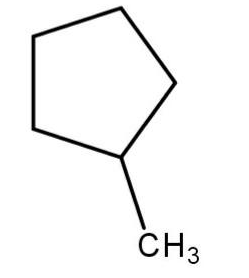
(a)

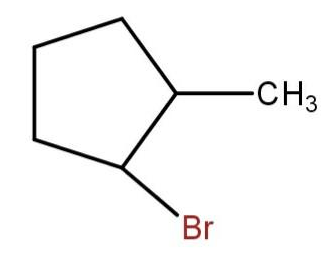
(b)
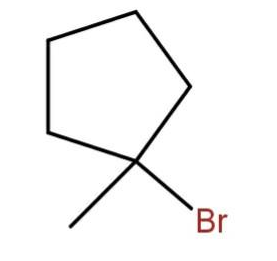
(c)
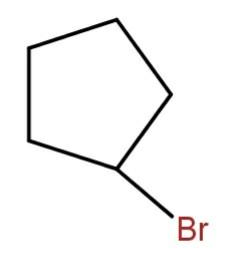
(d)





Answer
544.2k+ views
Hint: In these questions, we have to know the reaction of a given structure with $ B{r_2} $ in presence of the light and then the stability of which structure is maximum, will be the major product. The stability will depend on the intermediate.
Complete step by step solution:
Here, first we start with the reaction of the given structure with $ B{r_2} $ in the presence of light:
In these reactions, first the bromine forms free radical when exposed to the sunlight. The bromine will replace the hydrogen from that place, where the removal of hydrogen makes the carbocation most stable.

As the tertiary carbon is the most stable carbocation. So, the bromine will attach to the tertiary carbon.
Hence, the major product will be
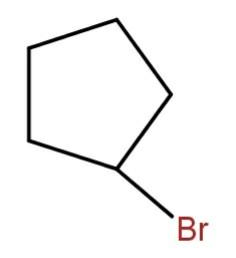
Hence, the correct option is (d).
Additional Information:
Carbocation: The carbocation is a molecule which is having a positive charge and three bonded atoms.
A carbocation is formed as an intermediate in a reaction, with the loss of one electron.
The carbocation are of three types:
(a) Primary Carbocation
(b) Secondary Carbocation
(c) Tertiary Carbocation
The definitions of different types of carbocation:
(a) Primary Carbocation: The carbocation in which carbon is bonded with two hydrogens and one carbon atom.
(b) Secondary Carbocation: The carbocation in which carbon is bonded with one hydrogen and two carbon atoms.
(c) Tertiary Carbocation: The carbocation in which carbon is bonded with three carbon atoms only.
Note:
When the reaction, there are formation of many minor products. Minor products are at all those positions where the carbocation can be formed. But the major will be the product, which has the highest stable carbocation.
Complete step by step solution:
Here, first we start with the reaction of the given structure with $ B{r_2} $ in the presence of light:
In these reactions, first the bromine forms free radical when exposed to the sunlight. The bromine will replace the hydrogen from that place, where the removal of hydrogen makes the carbocation most stable.

As the tertiary carbon is the most stable carbocation. So, the bromine will attach to the tertiary carbon.
Hence, the major product will be

Hence, the correct option is (d).
Additional Information:
Carbocation: The carbocation is a molecule which is having a positive charge and three bonded atoms.
A carbocation is formed as an intermediate in a reaction, with the loss of one electron.
The carbocation are of three types:
(a) Primary Carbocation
(b) Secondary Carbocation
(c) Tertiary Carbocation
The definitions of different types of carbocation:
(a) Primary Carbocation: The carbocation in which carbon is bonded with two hydrogens and one carbon atom.
(b) Secondary Carbocation: The carbocation in which carbon is bonded with one hydrogen and two carbon atoms.
(c) Tertiary Carbocation: The carbocation in which carbon is bonded with three carbon atoms only.
Note:
When the reaction, there are formation of many minor products. Minor products are at all those positions where the carbocation can be formed. But the major will be the product, which has the highest stable carbocation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




