
What is the shape of d- orbital?
A. Spherical
B. Dumbbell
C. Double dumbbell
D. No definite shape
Answer
547.8k+ views
Hint: Shape of orbitals are essentially the share of electron wave shapes and are named as s, p, d, f basis of the observations of line spectra. Also, rather than saying that the electron wave has an s-shape, we say electron is available in s-orbital
Complete step by step answer:
As we know that the electron wave shapes are chosen based on observations of line spectra. The words s, p, d, f were chosen based on the observations as certain lines were observed as “sharp”, “principal”, “diffuse’, or “fundamental” series. Since, electrons exhibit both particle and wave nature, instead of saying “the electron wave has a d shape” we say “the electron is in a d orbital”.
Orbital is essential as the wave function of an electron and is defined by the three quantum numbers energy (n), angular momentum (l), and Magnetic moment (ml) and it describes the region in space having a high probability of finding the electron.
In the case of d-orbital, l=2 and the first principal shell to have a d subshell corresponds to n=3. The ml values for five d orbitals are -2, -1, 0, +1, and +2 i.e., we can say d-subshell has five orientations. All these d-orbitals have the same energy and are called degenerate orbitals. The shape of the d-orbitals is given below:
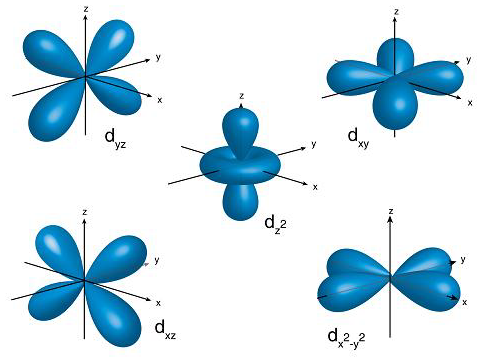
Hence, we can say d-orbitals have double dumbbell-shaped.
So, the correct answer is Option C.
Note: The maximum number of electrons for any subshell can be calculated using the azimuthal quantum number (l) is $2(2l + 1)$. So for the case of s-orbital l=0 and hence it can have only 2 electrons. For the case of l-orbital, l=1 and the maximum number of electrons is 6, similarly, for d-orbital, l=2 and maximum 10 electrons can be present, and p-orbital with l=3 can hold maximum 14 electrons
Complete step by step answer:
As we know that the electron wave shapes are chosen based on observations of line spectra. The words s, p, d, f were chosen based on the observations as certain lines were observed as “sharp”, “principal”, “diffuse’, or “fundamental” series. Since, electrons exhibit both particle and wave nature, instead of saying “the electron wave has a d shape” we say “the electron is in a d orbital”.
Orbital is essential as the wave function of an electron and is defined by the three quantum numbers energy (n), angular momentum (l), and Magnetic moment (ml) and it describes the region in space having a high probability of finding the electron.
In the case of d-orbital, l=2 and the first principal shell to have a d subshell corresponds to n=3. The ml values for five d orbitals are -2, -1, 0, +1, and +2 i.e., we can say d-subshell has five orientations. All these d-orbitals have the same energy and are called degenerate orbitals. The shape of the d-orbitals is given below:

Hence, we can say d-orbitals have double dumbbell-shaped.
So, the correct answer is Option C.
Note: The maximum number of electrons for any subshell can be calculated using the azimuthal quantum number (l) is $2(2l + 1)$. So for the case of s-orbital l=0 and hence it can have only 2 electrons. For the case of l-orbital, l=1 and the maximum number of electrons is 6, similarly, for d-orbital, l=2 and maximum 10 electrons can be present, and p-orbital with l=3 can hold maximum 14 electrons
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




