
Shape of $Fe{(CO)_5}$ is:
A. Octahedral
B. Square planar
C. trigonal bipyramidal
D. square pyramidal
Answer
569.7k+ views
Hint: In $Fe{(CO)_5}$, the central metal atom is Fe and ligand is carbon monoxide. The central metal atom Fe is surrounded by five carbon monoxide ligands. The Carbon monoxide ligand is a strong field ligand so it will pull the electrons of iron towards itself to form a low spin complex.
Complete step by step answer:
The chemical name of the compound $Fe{(CO)_5}$is iron pentacarbonyl.
The atomic number of iron Fe is 26. The oxidation state of iron (Fe) in iron pentacarbonyl($Fe{(CO)_5}$) is 0.
Thus, the electronic configuration of Fe is $[Ar]3{d^6}4{s^2}$.
In the complex $Fe{(CO)_5}$, Fe is a central metal atom and carbon monoxide CO is a ligand surrounding the central metal atom.
The ligand CO is a strong field ligand due to the presence of pi- back bonding, so it will push the electrons of the central metal towards itself to generate a low spin complex.
The six electrons of the 3d orbital pair up with each other and the electrons of 4s orbital shift to the 3d orbital leaving an empty orbital.
The molecular orbital diagram for the formation of $Fe{(CO)_5}$is shown below.
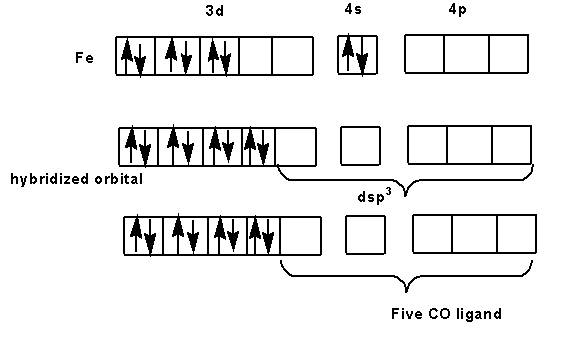
After the pairing of electrons, one 3d orbital, one 4s orbital and three 4p orbital are empty in which five carbon monoxide ligands bind to form $Fe{(CO)_5}$complex. Here, one 3d orbital, one 4s orbital and three 4p orbital combine to form $ds{p^3}$ hybridization.Thus, the shape of the molecule will be trigonal bipyramidal.
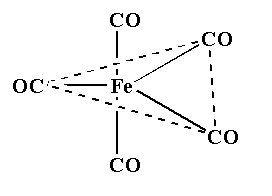
The structure is shown below.
So, the correct answer is “Option C”.
Note:
The complex where metal is surrounded by carbon monoxide ligand is also called metal carbonyl which is a coordination complex.
Complete step by step answer:
The chemical name of the compound $Fe{(CO)_5}$is iron pentacarbonyl.
The atomic number of iron Fe is 26. The oxidation state of iron (Fe) in iron pentacarbonyl($Fe{(CO)_5}$) is 0.
Thus, the electronic configuration of Fe is $[Ar]3{d^6}4{s^2}$.
In the complex $Fe{(CO)_5}$, Fe is a central metal atom and carbon monoxide CO is a ligand surrounding the central metal atom.
The ligand CO is a strong field ligand due to the presence of pi- back bonding, so it will push the electrons of the central metal towards itself to generate a low spin complex.
The six electrons of the 3d orbital pair up with each other and the electrons of 4s orbital shift to the 3d orbital leaving an empty orbital.
The molecular orbital diagram for the formation of $Fe{(CO)_5}$is shown below.

After the pairing of electrons, one 3d orbital, one 4s orbital and three 4p orbital are empty in which five carbon monoxide ligands bind to form $Fe{(CO)_5}$complex. Here, one 3d orbital, one 4s orbital and three 4p orbital combine to form $ds{p^3}$ hybridization.Thus, the shape of the molecule will be trigonal bipyramidal.
The structure is shown below.

So, the correct answer is “Option C”.
Note:
The complex where metal is surrounded by carbon monoxide ligand is also called metal carbonyl which is a coordination complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




