
What is the shape of orbitals ${{d}_{xy}},{{d}_{xz,}}{{d}_{yz,}}{{d}_{{{x}^{2}}-{{y}^{2}},}}{{d}_{{{z}^{2}}}}$
Answer
558.9k+ views
Hint: Five orbitals are represented with respect to the coordinate axis in which the orbitals are placed.
- One of the d-orbitals is different from the remaining orbitals.
Complete step by step answer:
So in the question we are asked to comment on the shape of the five d-orbitals i.e. ${{d}_{xy}},{{d}_{xz,}}{{d}_{yz,}}{{d}_{{{x}^{2}}-{{y}^{2}},}}{{d}_{{{z}^{2}}}}$
First let’s look at what the orbital means in chemistry.
- If we take the atomic theory and quantum mechanical approach of an atom, the orbital is the mathematical function which describes the location or position of an electron and explains the wave-like behavior of electrons in the atom.
- And this function is used for the calculation of the probability of finding electrons in the specific area in an atom. Orbitals are referred to as the three dimensional area near the nucleus of an atom where we have the possibility of finding electrons.
- Each orbitals have its own unique set of quantum numbers for n, l, m and s, which gives the energy, angular momentum, magnetic momentum and spin of the electron in the orbital.
- Now let’s see about the d-orbitals.
d-orbitals are introduced from the third shell i.e. with n value as 3.
If n = 3 .we know that,
l (angular momentum quantum number)= (n - 1) = 3 - 1 = 2, which represents the d-orbitals.
And if value of l is 2 then m, magnetic quantum number which gives the number of orbitals have the value from,
m = -l to +l i.e. -2 to +2 values which consists of -2,-1.0, 1 and 2
So in total there are five d-orbitals.
- Now let’s talk about the shape of the d-orbitals-
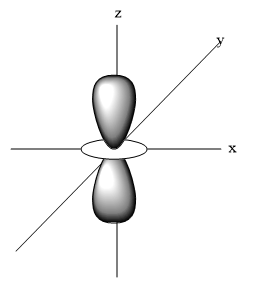
- Four orbitals of the d-orbitals are having double dumbbell shape whereas one of the d-orbital is different from the four and possessing a single dumbbell shaped structure.
-The four orbitals having double-dumbbell shapes are ${{d}_{xy}},{{d}_{xz,}}{{d}_{yz}} and \,{{d}_{{{x}^{2}}-{{y}^{2}}}}$ and the other one ${{d}_{{{z}^{2}}}}$ is having a single –dumbbell shaped structure.
The orbital’s subscript gives the idea of in which coordinate plane the orbitals are found and the probability of finding the electron.
The shape of the d orbitals are as follows,
- Shape of ${{d}_{xy}}$
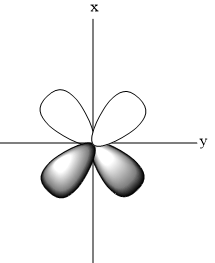
- Shape of ${{d}_{\begin{smallmatrix}
zx \\
\end{smallmatrix}}}$
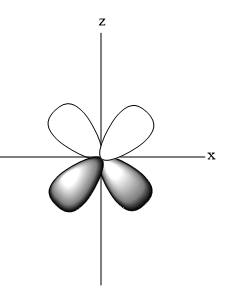
- Shape of ${{d}_{\begin{smallmatrix}
yz \\
\end{smallmatrix}}}$
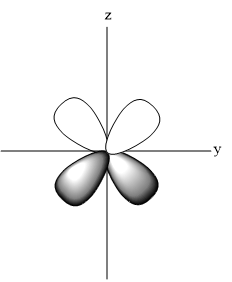
- Shape of ${{d}_{{{x}^{2}}-{{y}^{2}}}}$
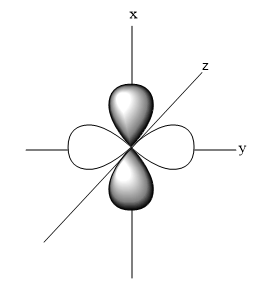
- Shape of ${{d}_{{{z}^{2}}}}$
So the correct answer is “a”:
Note: All the d-orbitals are degenerate in nature i.e. all the d-orbitals have the same energy and if any ligands approach them they are split into two sets -${{t}_{2}}_{g}\, and \,{{e}_{g}}$.
- One of the d-orbitals is different from the remaining orbitals.
Complete step by step answer:
So in the question we are asked to comment on the shape of the five d-orbitals i.e. ${{d}_{xy}},{{d}_{xz,}}{{d}_{yz,}}{{d}_{{{x}^{2}}-{{y}^{2}},}}{{d}_{{{z}^{2}}}}$
First let’s look at what the orbital means in chemistry.
- If we take the atomic theory and quantum mechanical approach of an atom, the orbital is the mathematical function which describes the location or position of an electron and explains the wave-like behavior of electrons in the atom.
- And this function is used for the calculation of the probability of finding electrons in the specific area in an atom. Orbitals are referred to as the three dimensional area near the nucleus of an atom where we have the possibility of finding electrons.
- Each orbitals have its own unique set of quantum numbers for n, l, m and s, which gives the energy, angular momentum, magnetic momentum and spin of the electron in the orbital.
- Now let’s see about the d-orbitals.
d-orbitals are introduced from the third shell i.e. with n value as 3.
If n = 3 .we know that,
l (angular momentum quantum number)= (n - 1) = 3 - 1 = 2, which represents the d-orbitals.
And if value of l is 2 then m, magnetic quantum number which gives the number of orbitals have the value from,
m = -l to +l i.e. -2 to +2 values which consists of -2,-1.0, 1 and 2
So in total there are five d-orbitals.
- Now let’s talk about the shape of the d-orbitals-
- Four orbitals of the d-orbitals are having double dumbbell shape whereas one of the d-orbital is different from the four and possessing a single dumbbell shaped structure.
-The four orbitals having double-dumbbell shapes are ${{d}_{xy}},{{d}_{xz,}}{{d}_{yz}} and \,{{d}_{{{x}^{2}}-{{y}^{2}}}}$ and the other one ${{d}_{{{z}^{2}}}}$ is having a single –dumbbell shaped structure.
The orbital’s subscript gives the idea of in which coordinate plane the orbitals are found and the probability of finding the electron.
The shape of the d orbitals are as follows,
- Shape of ${{d}_{xy}}$

- Shape of ${{d}_{\begin{smallmatrix}
zx \\
\end{smallmatrix}}}$

- Shape of ${{d}_{\begin{smallmatrix}
yz \\
\end{smallmatrix}}}$

- Shape of ${{d}_{{{x}^{2}}-{{y}^{2}}}}$

- Shape of ${{d}_{{{z}^{2}}}}$

So the correct answer is “a”:
Note: All the d-orbitals are degenerate in nature i.e. all the d-orbitals have the same energy and if any ligands approach them they are split into two sets -${{t}_{2}}_{g}\, and \,{{e}_{g}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




