
Show the formation of $N{a_2}O$ by transfer of electrons.
Answer
589.8k+ views
Hint: We can define transfer of electrons as a process in which an electron shares one or more electrons to its neighboring atom. We know that there must be eight electrons in the outermost orbital of an atom. This is known as the octet rule. If an atom has less than eight electrons, they tend to react and yield stable compounds.
Complete step by step answer:
We can state octet rule, as “An atom is more stable when their outermost shells are filled with eight electrons”. Molecules such as halogens, oxygen, nitrogen and carbon obey the octet rule. All the elements of the main group obey the octet rule.
Atoms are unstable in single form, except noble gases as their outermost electrons are completely filled. In order to be stable, atoms combine with each other.
We know that there are two main types of bonds. They are,
1.Ionic bonds
2.Covalent bonds
Ionic bonds are formed due to the transfer of electrons from one atom to another. This generally happens in metal. Ionic compounds such sodium chloride, potassium chloride, potassium nitrate, calcium chloride etc have ionic bonding in between their atoms.
Covalent bonds are formed due to the mutual sharing of electrons between two atoms. This is seen in non-metals. Covalent compounds such as water, hydrogen chloride, sulfur dioxide, carbon dioxide, methane have covalent bonds.
Now let us see the formation of $N{a_2}O$ due to transfer of electrons,
$N{a_2}O$ is an ionic compound and so the formation takes place due to transfer of electrons from sodium to oxygen atom.
We can draw the Lewis structure of $N{a_2}O$

From the Lewis structure, we can observe that oxygen needs two electrons to have filled valence orbitals. Sodium transfers one electron to oxygen since it needs two electrons to fulfill its octet.
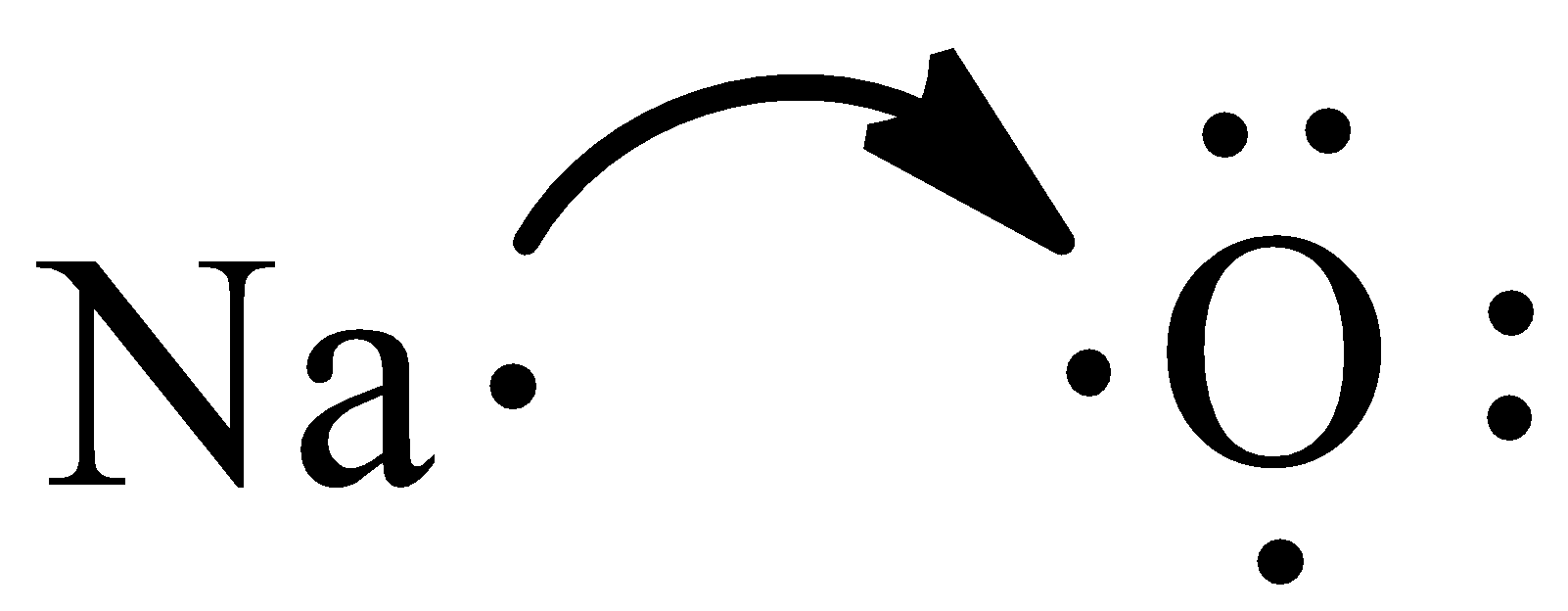
Oxygen requires one more electron to fulfill the octet. Therefore, another atom of sodium donates its electron to the oxygen atom.
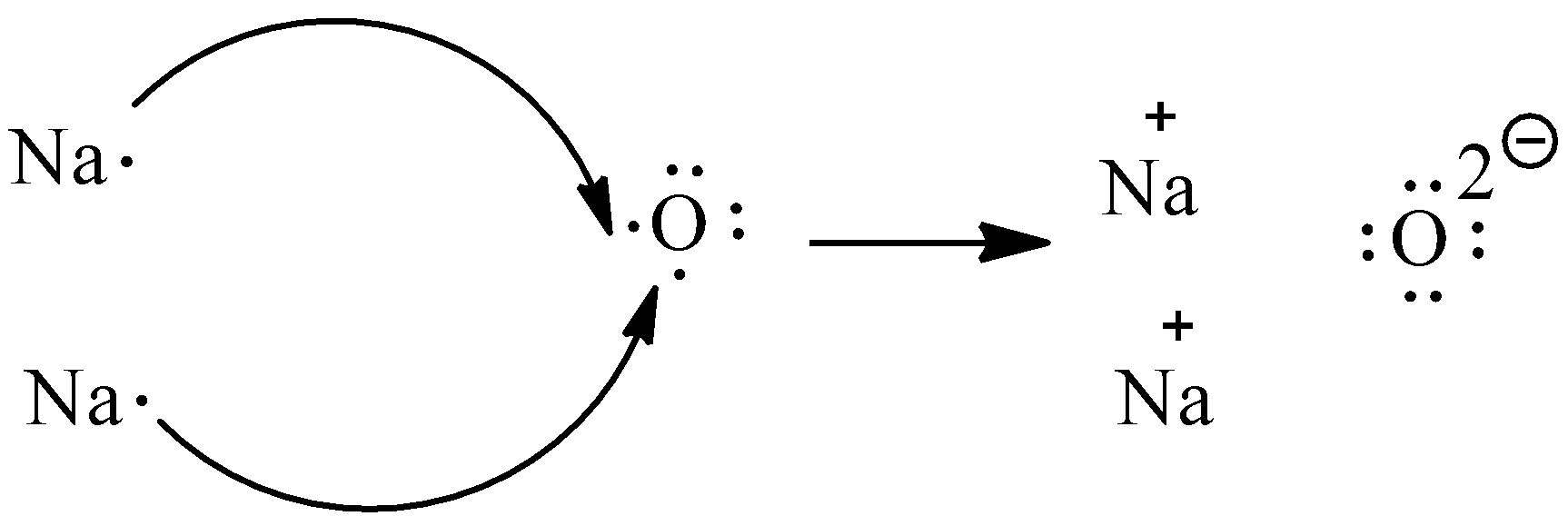
The overall neutral-charge ionic compound is given by three ions that attract one another. In an ionic compound, the number of electrons that are lost is equal to the number of electrons they gained.
Note:
Some of the compounds that do not obey octet rule are hydrogen, phosphorus, sulfur, lithium.
Example: The formation of calcium chloride takes place through the transfer of electrons.
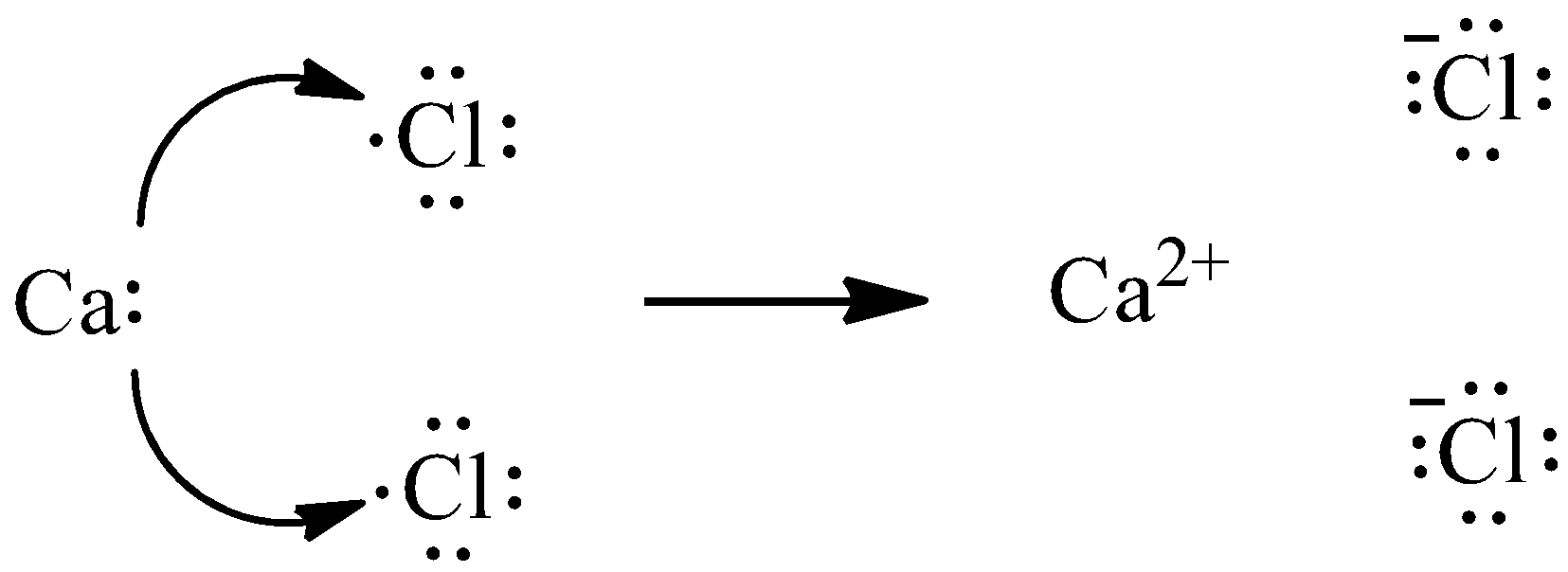
We know that calcium has two outermost electrons and chlorine has seven valence electrons. Chlorine requires only one electron to fulfill its octet, whereas calcium has two electrons to lose. Thus, we need two atoms of chlorine to gain two electrons from one atom of calcium. We can give the transfer process as,
Complete step by step answer:
We can state octet rule, as “An atom is more stable when their outermost shells are filled with eight electrons”. Molecules such as halogens, oxygen, nitrogen and carbon obey the octet rule. All the elements of the main group obey the octet rule.
Atoms are unstable in single form, except noble gases as their outermost electrons are completely filled. In order to be stable, atoms combine with each other.
We know that there are two main types of bonds. They are,
1.Ionic bonds
2.Covalent bonds
Ionic bonds are formed due to the transfer of electrons from one atom to another. This generally happens in metal. Ionic compounds such sodium chloride, potassium chloride, potassium nitrate, calcium chloride etc have ionic bonding in between their atoms.
Covalent bonds are formed due to the mutual sharing of electrons between two atoms. This is seen in non-metals. Covalent compounds such as water, hydrogen chloride, sulfur dioxide, carbon dioxide, methane have covalent bonds.
Now let us see the formation of $N{a_2}O$ due to transfer of electrons,
$N{a_2}O$ is an ionic compound and so the formation takes place due to transfer of electrons from sodium to oxygen atom.
We can draw the Lewis structure of $N{a_2}O$

From the Lewis structure, we can observe that oxygen needs two electrons to have filled valence orbitals. Sodium transfers one electron to oxygen since it needs two electrons to fulfill its octet.

Oxygen requires one more electron to fulfill the octet. Therefore, another atom of sodium donates its electron to the oxygen atom.

The overall neutral-charge ionic compound is given by three ions that attract one another. In an ionic compound, the number of electrons that are lost is equal to the number of electrons they gained.
Note:
Some of the compounds that do not obey octet rule are hydrogen, phosphorus, sulfur, lithium.
Example: The formation of calcium chloride takes place through the transfer of electrons.
We know that calcium has two outermost electrons and chlorine has seven valence electrons. Chlorine requires only one electron to fulfill its octet, whereas calcium has two electrons to lose. Thus, we need two atoms of chlorine to gain two electrons from one atom of calcium. We can give the transfer process as,

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




