
Show with the help of a diagram, the tetravalency of carbon.
Answer
565.8k+ views
Hint: Carbon is an element with an atomic number of 6.
- Carbon is a place with group-14 elements which possess a common oxidation state of +4.
- It possesses four valence electrons in their valence shell.
Complete Solution :
So in the question, it is asked that we have a comment on the tetravalency of carbon with a diagram. In the lower classes we have studies about elements in the periodic table, what is an atomic number and with atomic number how can we predict the electronic configuration of the element. And also how can we predict the number valence electrons and oxidation state of an element.
- Let’s approach this question with some basic ideas acquired in the earlier classes.
So we know that carbon is a chemical element which is represented as C. Carbon has an atomic number 6 i.e. it has 6 electrons and 6 protons in them. And its atomic mass is 12, hence the number of neutrons is also equal to 6.
- Now we can write the electronic configuration of C.
Electronic configuration of C = $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$
So from the electronic configuration we know that there are four valence electrons in the outermost shell i.e. the valence shell of the carbon atom.
- Hence the carbon atom can either share or lose these four electrons to obtain the noble gas configuration and can also gain four more electrons to get the noble gas or stable octet configuration. Hence the carbon should be tetravalent to do so.
- Tetravalency means that an element could extend its valency only upto four or the atom could only form four bonds with other atoms. Here, since C shares four electrons to form four covalent bonds with other atoms it is a tetravalent atom.
- Generally the C shares the four electrons with other four electrons and only forms the covalent bonds.
- Now let’s draw the Lewis structure of methane. In the case of $C{{H}_{4}}$, the hybridisation of the central atom carbon is $s{{p}^{3}}$. It forms four sigma bonds with four hydrogens around it. The bond angle between any two hydrogens is $109.5^o$ The diagram depicting the tetravalency of carbon is shown below:
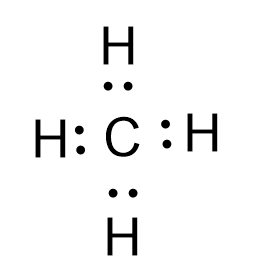
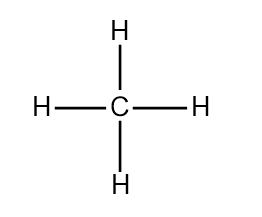
Here by the sharing of electrons by C and H atoms the central atom carbon attains the octet configuration and each H atom also obtains the stable noble gas configuration of He.
- Carbon cannot extend its valency to more than four.
Note: Remember that the valency of an atom is independent of its hybridisation. If the carbon atom is $s{{p}^{2}}$ hybridised, then the number of sigma bonds will change, but the total bonds will remain the same i.e. four.
- Carbon is a place with group-14 elements which possess a common oxidation state of +4.
- It possesses four valence electrons in their valence shell.
Complete Solution :
So in the question, it is asked that we have a comment on the tetravalency of carbon with a diagram. In the lower classes we have studies about elements in the periodic table, what is an atomic number and with atomic number how can we predict the electronic configuration of the element. And also how can we predict the number valence electrons and oxidation state of an element.
- Let’s approach this question with some basic ideas acquired in the earlier classes.
So we know that carbon is a chemical element which is represented as C. Carbon has an atomic number 6 i.e. it has 6 electrons and 6 protons in them. And its atomic mass is 12, hence the number of neutrons is also equal to 6.
- Now we can write the electronic configuration of C.
Electronic configuration of C = $1{{s}^{2}}2{{s}^{2}}2{{p}^{2}}$
So from the electronic configuration we know that there are four valence electrons in the outermost shell i.e. the valence shell of the carbon atom.
- Hence the carbon atom can either share or lose these four electrons to obtain the noble gas configuration and can also gain four more electrons to get the noble gas or stable octet configuration. Hence the carbon should be tetravalent to do so.
- Tetravalency means that an element could extend its valency only upto four or the atom could only form four bonds with other atoms. Here, since C shares four electrons to form four covalent bonds with other atoms it is a tetravalent atom.
- Generally the C shares the four electrons with other four electrons and only forms the covalent bonds.
- Now let’s draw the Lewis structure of methane. In the case of $C{{H}_{4}}$, the hybridisation of the central atom carbon is $s{{p}^{3}}$. It forms four sigma bonds with four hydrogens around it. The bond angle between any two hydrogens is $109.5^o$ The diagram depicting the tetravalency of carbon is shown below:


Here by the sharing of electrons by C and H atoms the central atom carbon attains the octet configuration and each H atom also obtains the stable noble gas configuration of He.
- Carbon cannot extend its valency to more than four.
Note: Remember that the valency of an atom is independent of its hybridisation. If the carbon atom is $s{{p}^{2}}$ hybridised, then the number of sigma bonds will change, but the total bonds will remain the same i.e. four.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




