
How many sigma and pi bonds are in the HCN molecule?
Answer
534.3k+ views
Hint: Hydrogen cyanide is an acid that has molecular formula $H-C\equiv N$. We know that when a pair of electrons are shared by two atoms, a covalent bond is formed. Sigma ($\sigma $) bonds and pi ($\pi $) bonds are two types of covalent bonds.
Complete step by step solution:
The strongest type of covalent chemical bond is a sigma ($\sigma $) bond. A sigma ($\sigma $) bond is formed when the atomic orbitals overlap head-on. Usually, sigma ($\sigma $) bonds are single bonds.
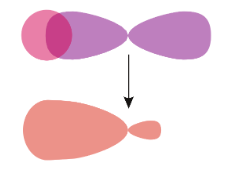
The sigma ($\sigma $) bonds are symmetrical and can rotate about the bond axis. Some of the most common sigma bonds are s+s, ${{p}_{z}}+{{p}_{z}}$, s+${{p}_{z}}$, and ${{d}_{{{z}^{2}}}}+{{d}_{{{z}^{2}}}}$. Here, s, p, and d are atomic orbitals and z is the bond axis.
Now, the covalent chemical bonds which are formed when the atomic orbitals overlap laterally are known as pi ($\pi $) bonds. Usually, there is 1 pi ($\pi $) bond in double bonds and 2 pi ($\pi $) bonds in triple bonds.
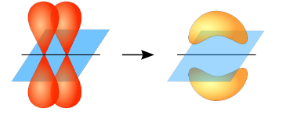
Pi ($\pi $) bonds cannot rotate about the bond axis without breaking the bond.
Now, in HCN, we can see that there are two single bonds, H-C and C-N, hence it has two sigma ($\sigma $) bonds. Whereas it has a triple bond in $C\equiv N$ and hence has two pi ($\pi $) bonds.
So, the HCN molecule has 2 sigma ($\sigma $) bonds and 2 pi ($\pi $) bonds.
Additional Information: Some of the properties of HCN are
- It can be synthesized by combining ammonia and methane
\[2C{{H}_{4}}+2N{{H}_{3}}+3{{O}_{2}}\xrightarrow[\Delta =1200{}^\circ C]{Pt}2HCN+6{{H}_{2}}O\]
- It has an odor of bitter almond oil.
- It exists in both the gaseous as well as the liquid state.
- It has a melting point of 259.86 K and a boiling point of 299.00 K.
- It has a density of 0.69 g/l.
- It has a linear structure.
- It is extremely toxic as well as poisonous in nature and has been used as chemical weapons.
- It is colorless and is soluble and miscible in water and ethanol.
Note: It should be noted that even though pi ($\pi $) bonds are weaker than sigma ($\sigma $) bonds, but when atoms are bonded by both sigma ($\sigma $) bonds and pi ($\pi $) bonds, their strength is greater than either of the bonds alone. Hence the strength of multiple bonds (double and triple bonds) is more than that of a single bond.
Complete step by step solution:
The strongest type of covalent chemical bond is a sigma ($\sigma $) bond. A sigma ($\sigma $) bond is formed when the atomic orbitals overlap head-on. Usually, sigma ($\sigma $) bonds are single bonds.

The sigma ($\sigma $) bonds are symmetrical and can rotate about the bond axis. Some of the most common sigma bonds are s+s, ${{p}_{z}}+{{p}_{z}}$, s+${{p}_{z}}$, and ${{d}_{{{z}^{2}}}}+{{d}_{{{z}^{2}}}}$. Here, s, p, and d are atomic orbitals and z is the bond axis.
Now, the covalent chemical bonds which are formed when the atomic orbitals overlap laterally are known as pi ($\pi $) bonds. Usually, there is 1 pi ($\pi $) bond in double bonds and 2 pi ($\pi $) bonds in triple bonds.

Pi ($\pi $) bonds cannot rotate about the bond axis without breaking the bond.
Now, in HCN, we can see that there are two single bonds, H-C and C-N, hence it has two sigma ($\sigma $) bonds. Whereas it has a triple bond in $C\equiv N$ and hence has two pi ($\pi $) bonds.
So, the HCN molecule has 2 sigma ($\sigma $) bonds and 2 pi ($\pi $) bonds.
Additional Information: Some of the properties of HCN are
- It can be synthesized by combining ammonia and methane
\[2C{{H}_{4}}+2N{{H}_{3}}+3{{O}_{2}}\xrightarrow[\Delta =1200{}^\circ C]{Pt}2HCN+6{{H}_{2}}O\]
- It has an odor of bitter almond oil.
- It exists in both the gaseous as well as the liquid state.
- It has a melting point of 259.86 K and a boiling point of 299.00 K.
- It has a density of 0.69 g/l.
- It has a linear structure.
- It is extremely toxic as well as poisonous in nature and has been used as chemical weapons.
- It is colorless and is soluble and miscible in water and ethanol.
Note: It should be noted that even though pi ($\pi $) bonds are weaker than sigma ($\sigma $) bonds, but when atoms are bonded by both sigma ($\sigma $) bonds and pi ($\pi $) bonds, their strength is greater than either of the bonds alone. Hence the strength of multiple bonds (double and triple bonds) is more than that of a single bond.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




