
Sodium benzoate is converted to benzene by heating with anhydrous ferric chloride.
A) True
B) False
Answer
524.3k+ views
Hint: We know that sodium benzoate is $ - {\text{COONa}}$ group attached to the benzene ring. To solve this we must know the reagent used to convert sodium benzoate to benzene. We must know whether anhydrous ferric chloride reacts with sodium benzoate or not.
Complete answer:Sodium benzoate can be converted to benzene by heating it with a mixture of sodium hydroxide $\left( {{\text{NaOH}}} \right)$ and calcium oxide $\left( {{\text{CaO}}} \right)$.
When sodium benzoate is heated with a mixture of sodium hydroxide and calcium oxide benzene is produced. Along with benzene sodium carbonate is also produced as the by-product.
The mixture of sodium hydroxide and calcium oxide is known as soda lime. The chemical formula for soda lime is ${\text{CaHNa}}{{\text{O}}_{\text{2}}}$.
The reaction of sodium benzoate with a mixture of sodium hydroxide and calcium oxide is as follows:
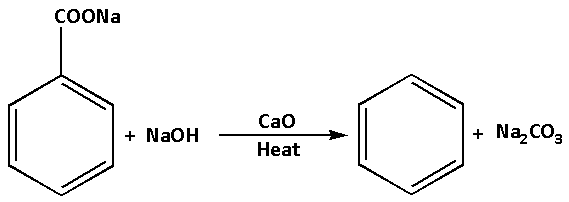
The reaction in which carboxyl groups are removed or carbon dioxide is removed is known as decarboxylation reaction. Thus, the reaction of sodium benzoate with a mixture of sodium hydroxide and calcium oxide is a decarboxylation reaction. This is because in the reaction, the carboxyl group is removed.
Thus, sodium benzoate to produce benzene reacts with a mixture of sodium hydroxide and calcium oxide and not with anhydrous ferric chloride.
Thus, the statement ‘sodium benzoate is converted to benzene by heating with anhydrous ferric chloride’ is false.
Thus, the correct option is (B) false.
Note: Ferric chloride $\left( {{\text{FeC}}{{\text{l}}_{\text{3}}}} \right)$ solution is light brown to colourless in appearance and smells like hydrochloric acid. The anhydrous ferric chloride needs electrons to complete its octet. Thus, anhydrous ferric chloride acts as a Lewis acid. The main function of anhydrous ferric chloride is to produce an electrophile.
Complete answer:Sodium benzoate can be converted to benzene by heating it with a mixture of sodium hydroxide $\left( {{\text{NaOH}}} \right)$ and calcium oxide $\left( {{\text{CaO}}} \right)$.
When sodium benzoate is heated with a mixture of sodium hydroxide and calcium oxide benzene is produced. Along with benzene sodium carbonate is also produced as the by-product.
The mixture of sodium hydroxide and calcium oxide is known as soda lime. The chemical formula for soda lime is ${\text{CaHNa}}{{\text{O}}_{\text{2}}}$.
The reaction of sodium benzoate with a mixture of sodium hydroxide and calcium oxide is as follows:

The reaction in which carboxyl groups are removed or carbon dioxide is removed is known as decarboxylation reaction. Thus, the reaction of sodium benzoate with a mixture of sodium hydroxide and calcium oxide is a decarboxylation reaction. This is because in the reaction, the carboxyl group is removed.
Thus, sodium benzoate to produce benzene reacts with a mixture of sodium hydroxide and calcium oxide and not with anhydrous ferric chloride.
Thus, the statement ‘sodium benzoate is converted to benzene by heating with anhydrous ferric chloride’ is false.
Thus, the correct option is (B) false.
Note: Ferric chloride $\left( {{\text{FeC}}{{\text{l}}_{\text{3}}}} \right)$ solution is light brown to colourless in appearance and smells like hydrochloric acid. The anhydrous ferric chloride needs electrons to complete its octet. Thus, anhydrous ferric chloride acts as a Lewis acid. The main function of anhydrous ferric chloride is to produce an electrophile.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




