
Sodium is a very reactive metal. Is this statement true or false?
Answer
508.8k+ views
Hint: The reactivity of an element is dependent on a lot of factors like its size, the ability to lose or gain electrons and the effective nuclear charge experienced by the valence electrons of the atom. A series of reactions and their observations has led to the formation of a reactivity series.
Complete answer:
Sodium is an alkali metal belonging to the first group of the periodic table. All alkali metals have a single electron in their valence shells. The considerably large sizes of alkali metals reduces the effective nuclear charge experienced by the valence electron which is responsible for their low ionization energies and high electropositivity.
A metal is said to be reactive if it is highly electropositive in nature and readily loses its electrons in a reaction. Sodium metal has very low ionization energy and therefore readily loses its electron.
\[Na(g) \to N{a^ + }(g) + {e^ - }{\text{ I}}{\text{.E = 496}}KJmo{l^{ - 1}}\]
Sodium reacts vigorously with oxygen and water releasing enormous amounts of energy (highly exothermic reactions) that it catches fire and explodes with popping sounds. Therefore it is always stored in kerosene as even little exposure results in hazardous reactions.
\[2Na(s) + 2{H_2}O(l) \to 2NaOH(aq) + {H_2}(g) + {\text{large amount of heat energy}}\]
Such intense reactions with water, air and acids shown by sodium make it the second most reactive metal in the reactivity series.
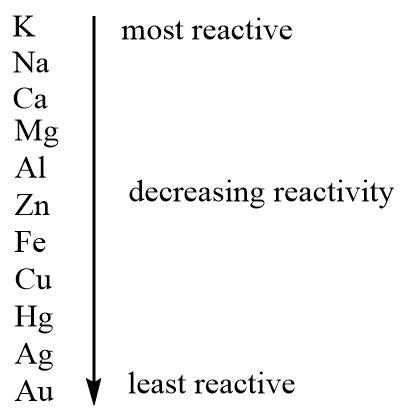
Hence it is true that sodium is a very reactive metal.
Note:
The reaction for ionization was written in the gas phase even though sodium is metal that exists in solid state because ionization enthalpy is always calculated when an element is in its ground state, gaseous form and completely isolated from other atoms or molecules.
Complete answer:
Sodium is an alkali metal belonging to the first group of the periodic table. All alkali metals have a single electron in their valence shells. The considerably large sizes of alkali metals reduces the effective nuclear charge experienced by the valence electron which is responsible for their low ionization energies and high electropositivity.
A metal is said to be reactive if it is highly electropositive in nature and readily loses its electrons in a reaction. Sodium metal has very low ionization energy and therefore readily loses its electron.
\[Na(g) \to N{a^ + }(g) + {e^ - }{\text{ I}}{\text{.E = 496}}KJmo{l^{ - 1}}\]
Sodium reacts vigorously with oxygen and water releasing enormous amounts of energy (highly exothermic reactions) that it catches fire and explodes with popping sounds. Therefore it is always stored in kerosene as even little exposure results in hazardous reactions.
\[2Na(s) + 2{H_2}O(l) \to 2NaOH(aq) + {H_2}(g) + {\text{large amount of heat energy}}\]
Such intense reactions with water, air and acids shown by sodium make it the second most reactive metal in the reactivity series.

Hence it is true that sodium is a very reactive metal.
Note:
The reaction for ionization was written in the gas phase even though sodium is metal that exists in solid state because ionization enthalpy is always calculated when an element is in its ground state, gaseous form and completely isolated from other atoms or molecules.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




