
How do you solve the gas law stoichiometry problem?
Answer
548.1k+ views
Hint: To solve the gas law stoichiometry problem we are supposed to know the number of moles of each substance involved in the process. Gas laws are going to help to determine the effect of physical parameters on the gas.
Complete step by step answer:
- In the question, it is asked how we can solve the gas law stoichiometry problem.
- Gas laws help us to determine the effect of pressure, temperature, and volume on the given number of moles of the gas.
- In any stoichiometry problem the main need is to convert the number of moles A into the number of moles B.
- We have to follow a few steps to solve the gas law stoichiometry problem and they are as follows.
1. Initially we have to balance the chemical reaction.
2. Convert the given mass of the reactants from grams to moles by using the molar mass of the reactant.
3. In the third step we have to get the molar ratio between the reactant to the product, it will give the number of moles of the product.
4. At last we have to use the molar volume to get the moles in liters for gaseous products.
- The below chart explains clearly the above steps to solve the gas law stoichiometry problem
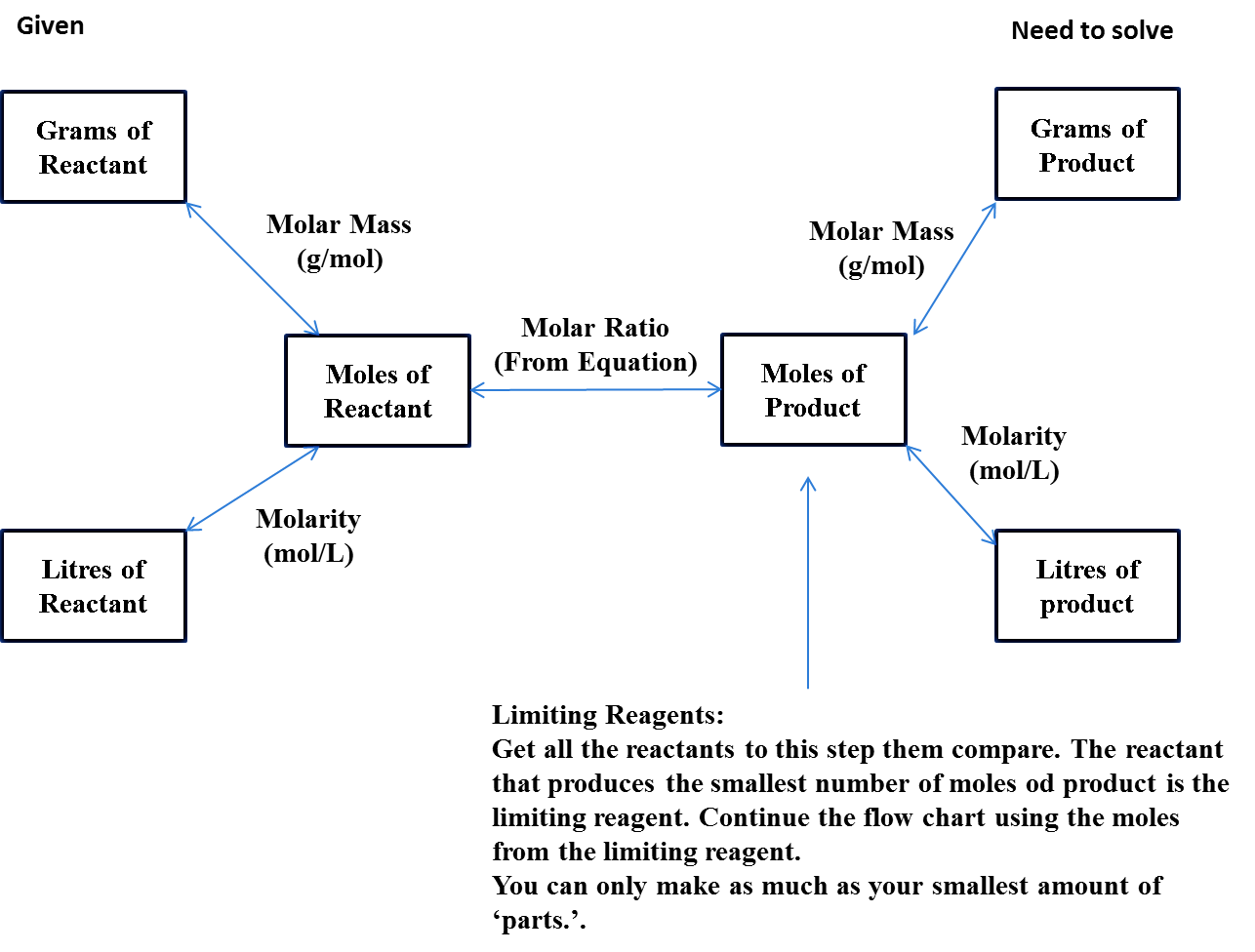
Note: If the reactant and product are solids or liquids we have to use the mass and molar mass to get the moles, if we use the reactant and product are gases then we have to use the ideal gas law to get them in moles.
Complete step by step answer:
- In the question, it is asked how we can solve the gas law stoichiometry problem.
- Gas laws help us to determine the effect of pressure, temperature, and volume on the given number of moles of the gas.
- In any stoichiometry problem the main need is to convert the number of moles A into the number of moles B.
- We have to follow a few steps to solve the gas law stoichiometry problem and they are as follows.
1. Initially we have to balance the chemical reaction.
2. Convert the given mass of the reactants from grams to moles by using the molar mass of the reactant.
3. In the third step we have to get the molar ratio between the reactant to the product, it will give the number of moles of the product.
4. At last we have to use the molar volume to get the moles in liters for gaseous products.
- The below chart explains clearly the above steps to solve the gas law stoichiometry problem

Note: If the reactant and product are solids or liquids we have to use the mass and molar mass to get the moles, if we use the reactant and product are gases then we have to use the ideal gas law to get them in moles.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




