
What is the spin $\left( {{m_s}} \right)$ quantum number of electrons $9$?
A.0
B.$ + \dfrac{1}{2}$
C.$2$
D.$ - \dfrac{1}{2}$

Answer
503.7k+ views
Hint: We have to know that Quantum numbers can be utilized to portray the direction and the motion of an electron in an atom. The quantum numbers of the electrons in a given atom, when consolidated, should consent to the Schrodinger equation.
Complete answer:
We have to know that the sequence of numbers used to portray the position and energy of the electron in a particle is called quantum numbers. There are four quantum numbers, specifically, principal quantum number, azimuthal quantum number, magnetic quantum number and spin quantum number.
Let us now decide about spin quantum numbers.
The electron turn quantum number is free of the upsides of n, l, and ml. The value of this number provides understanding into the direction in which the electron is turning, and ${m_s}$ is the symbol which is used to represent spin quantum numbers.
The value of ${m_s}$ offers understanding into the course in which the electron is turning. The potential values of the electron spin quantum number are $ - \dfrac{1}{2}$ and $ + \dfrac{1}{2}$
The positive value of ${m_s}$ suggests a vertical spin on the electron which is additionally hit 'spin up' and is signified by the image ↑. In the event that ${m_s}$has a negative value, the electron being referred to is said to have a descending twist, or a 'spin down', which is given by the image ↓.
The value of the electron spin quantum number decides if the particle being referred to can deliver a magnetic field. The worth of ${m_s}$ can be summed up to $ \pm \dfrac{1}{2}$.
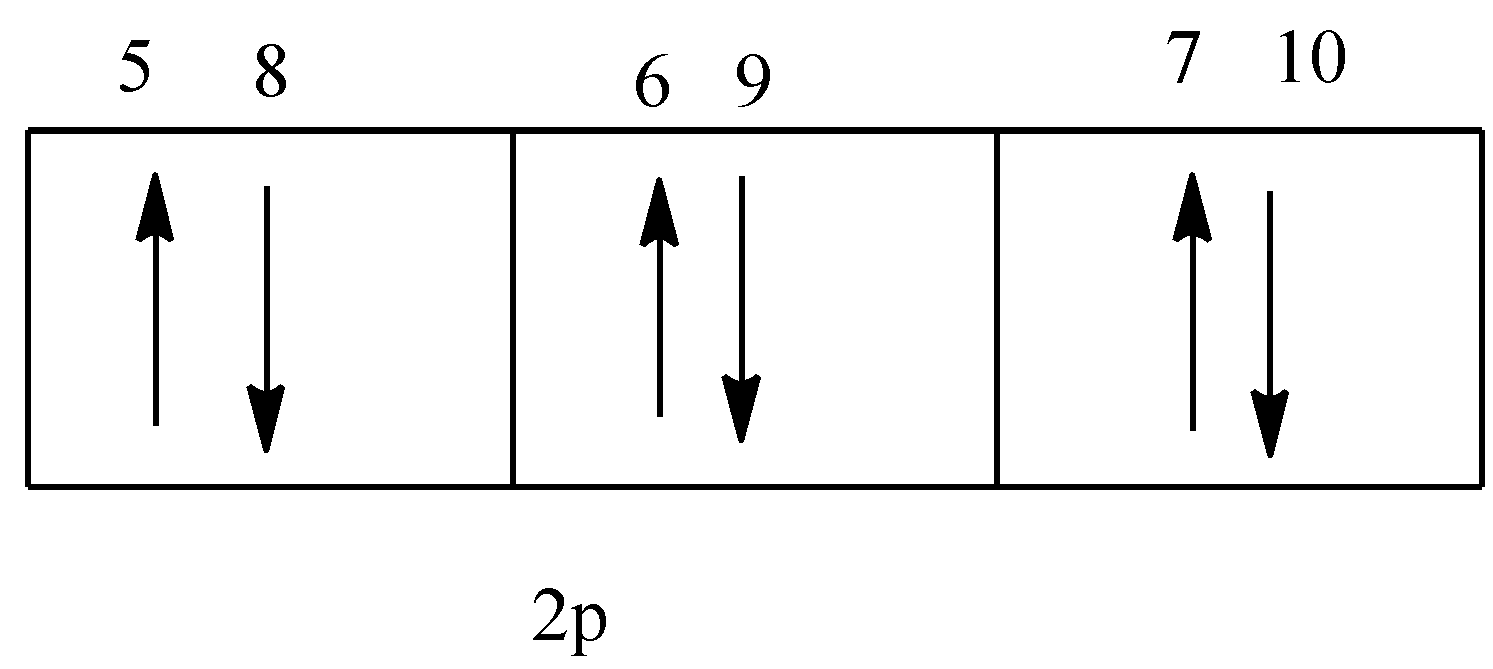
The given figure is,
In the given diagram, the electron 9 has down spin, so the value of spin quantum number will be $ - \dfrac{1}{2}$.
Option (D) is correct.
Note:
We have to know that for certain atoms, the spin of many unpaired electrons are taken together to give rise to produce a total spin quantum number, which is indicated by capital S. This is generally seen in light atoms if the spin-coupling is weaker than LS coupling.
Complete answer:
We have to know that the sequence of numbers used to portray the position and energy of the electron in a particle is called quantum numbers. There are four quantum numbers, specifically, principal quantum number, azimuthal quantum number, magnetic quantum number and spin quantum number.
Let us now decide about spin quantum numbers.
The electron turn quantum number is free of the upsides of n, l, and ml. The value of this number provides understanding into the direction in which the electron is turning, and ${m_s}$ is the symbol which is used to represent spin quantum numbers.
The value of ${m_s}$ offers understanding into the course in which the electron is turning. The potential values of the electron spin quantum number are $ - \dfrac{1}{2}$ and $ + \dfrac{1}{2}$
The positive value of ${m_s}$ suggests a vertical spin on the electron which is additionally hit 'spin up' and is signified by the image ↑. In the event that ${m_s}$has a negative value, the electron being referred to is said to have a descending twist, or a 'spin down', which is given by the image ↓.
The value of the electron spin quantum number decides if the particle being referred to can deliver a magnetic field. The worth of ${m_s}$ can be summed up to $ \pm \dfrac{1}{2}$.
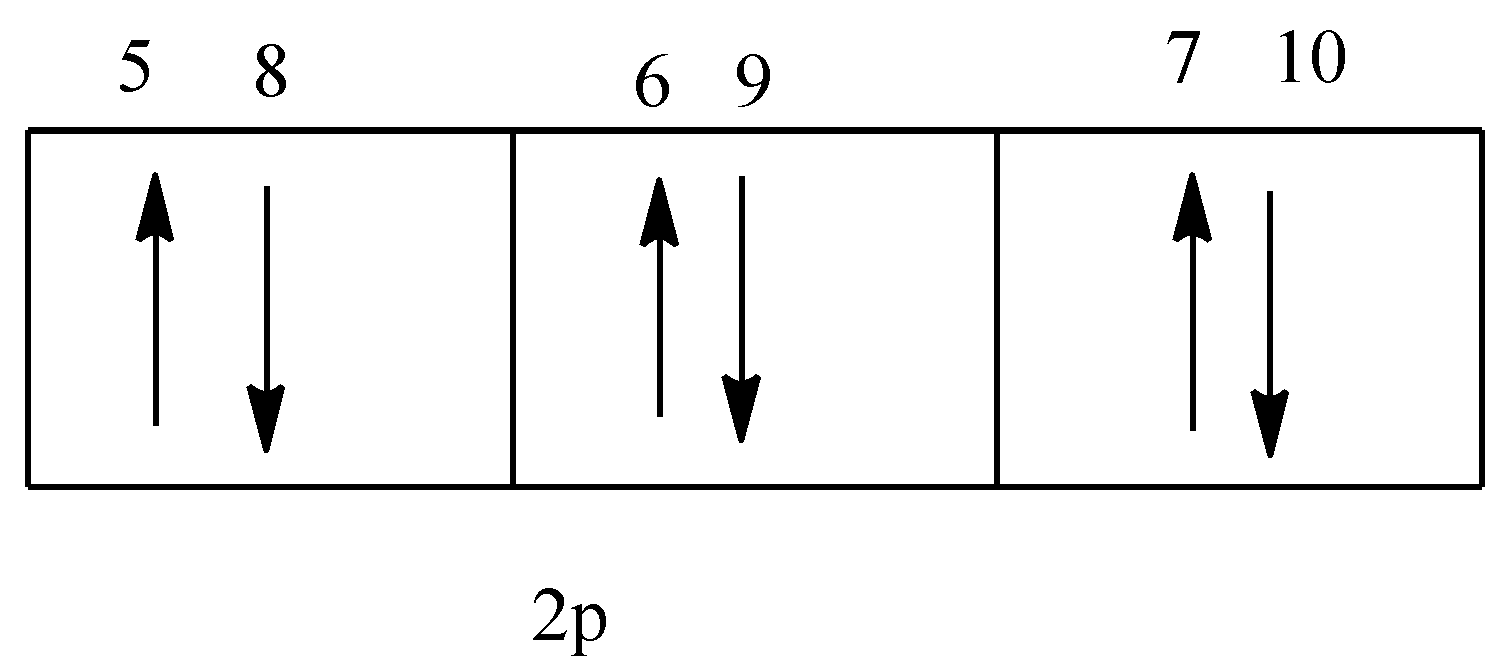
The given figure is,

In the given diagram, the electron 9 has down spin, so the value of spin quantum number will be $ - \dfrac{1}{2}$.
Option (D) is correct.
Note:
We have to know that for certain atoms, the spin of many unpaired electrons are taken together to give rise to produce a total spin quantum number, which is indicated by capital S. This is generally seen in light atoms if the spin-coupling is weaker than LS coupling.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




