
How many $S-S$ linkage (s) is /are present in ${{H}_{2}}{{S}_{4}}{{O}_{6}}$?
Answer
591.3k+ views
Hint: Construct the structure of ${{H}_{2}}{{S}_{4}}{{O}_{6}}$ by taking into consideration the valences of all the atoms and how they might be bonded to form a stable structure which is called tetrathionic acid.
Complete answer:
First, we will see what an $S-S$ linkage actually is; it is the sigma bond found between 2 atoms of sulphur. Now, if we arrange the given atoms in ${{H}_{2}}{{S}_{4}}{{O}_{6}}$ in the proper order, then we will get the number of $S-S$ bonds present.
We know that the valency of sulphur is 6, oxygen is 2, and hydrogen is 1. Thus, by arranging the atoms such that all the valences are satisfied and we get a structure that can be called tetrathionic acid, we get:
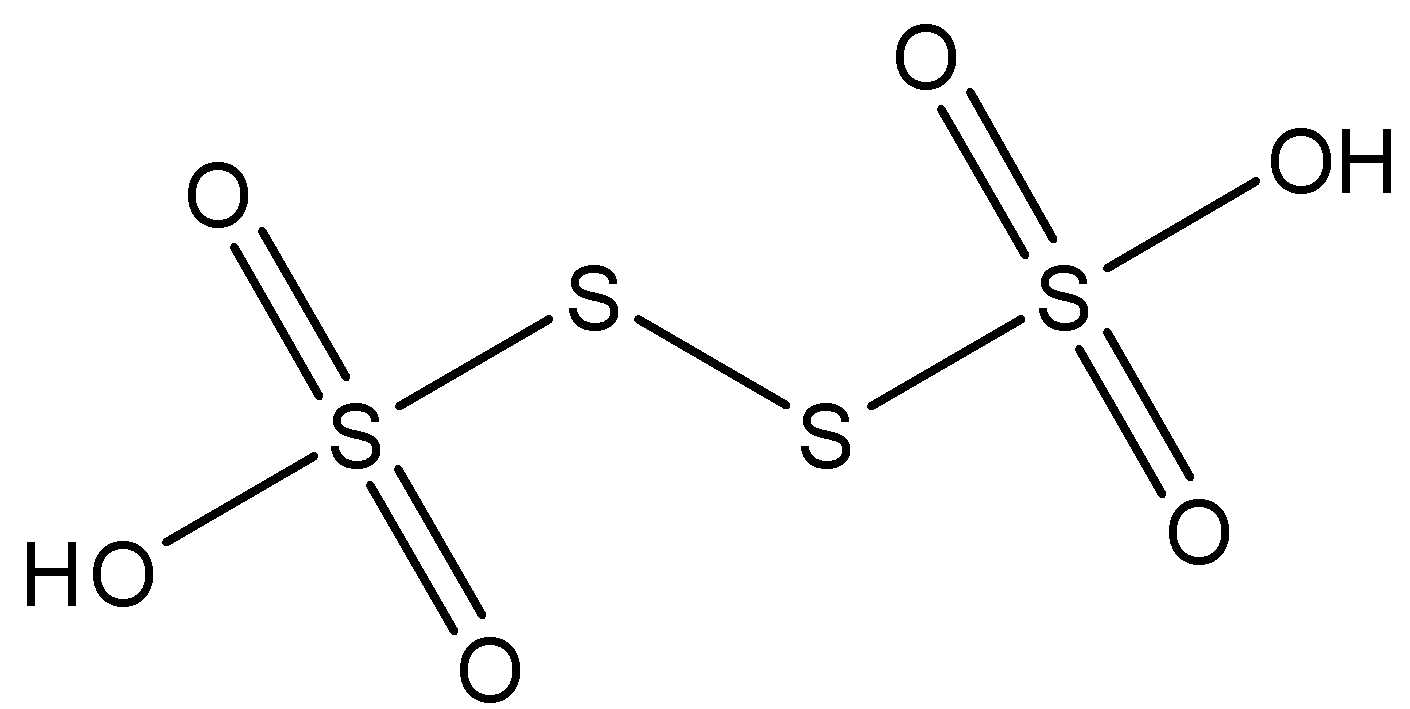
From the structure it is clear that ${{H}_{2}}{{S}_{4}}{{O}_{6}}$ is an oxo acid, which contains straight chain of sulphur atoms. This can be depicted from the formula ${{H}_{2}}{{S}_{n}}{{O}_{6}}$. We can also see the two acidic hydrogens in the hydroxyl groups that contribute to the name tetrathionic acid. We can clearly see that the number of $S-S$ bonds present are 3, in the general formula of oxo acids the number of $S-S$ bonds present will be $(n-1)$.
Therefore, 3 $S-S$ linkages are formed by ${{H}_{2}}{{S}_{4}}{{O}_{6}}$.
Note:
The oxidation state of 2 sulphur atoms in tetrathionic acid is +5. The oxidation state of the 2 central sulphur atoms in tetrathionic acid is 0. Thus, not all the sulphur atoms have the same oxidation state and the average oxidation state of sulphur will come out to be a fraction if we calculate it using usual methods. You can make use of this to predict the structure.
Complete answer:
First, we will see what an $S-S$ linkage actually is; it is the sigma bond found between 2 atoms of sulphur. Now, if we arrange the given atoms in ${{H}_{2}}{{S}_{4}}{{O}_{6}}$ in the proper order, then we will get the number of $S-S$ bonds present.
We know that the valency of sulphur is 6, oxygen is 2, and hydrogen is 1. Thus, by arranging the atoms such that all the valences are satisfied and we get a structure that can be called tetrathionic acid, we get:

From the structure it is clear that ${{H}_{2}}{{S}_{4}}{{O}_{6}}$ is an oxo acid, which contains straight chain of sulphur atoms. This can be depicted from the formula ${{H}_{2}}{{S}_{n}}{{O}_{6}}$. We can also see the two acidic hydrogens in the hydroxyl groups that contribute to the name tetrathionic acid. We can clearly see that the number of $S-S$ bonds present are 3, in the general formula of oxo acids the number of $S-S$ bonds present will be $(n-1)$.
Therefore, 3 $S-S$ linkages are formed by ${{H}_{2}}{{S}_{4}}{{O}_{6}}$.
Note:
The oxidation state of 2 sulphur atoms in tetrathionic acid is +5. The oxidation state of the 2 central sulphur atoms in tetrathionic acid is 0. Thus, not all the sulphur atoms have the same oxidation state and the average oxidation state of sulphur will come out to be a fraction if we calculate it using usual methods. You can make use of this to predict the structure.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




