
State octet rule.
Derive the Lewis dot structure of nitric acid molecules.
Answer
517.2k+ views
Hint: The chemical bonding in various chemical compounds is explained by the octet rule. It illustrates various bonds like ionic bonds, covalent bonds and coordinate bonds. But there are also exceptions to the octet rule.
Complete answer:
The octet rule states that every atom binds with another atom either by donating electrons or accepting electrons in its valence shell. According to this rule, a complete atom should have eight electrons in its outermost shell. The atoms that do not have complete orbitals will accept electrons and complete their valence shell to fulfill the octet rule. This rule explains the formation of many compounds. It also explains the stability of various compounds. Lewis structure is a structure that shows a covalent bond as a pair of electrons divided among the two molecules. Adding the number of outermost electrons in all the atoms in molecules gives the total number of valence electrons in the molecule. In this structure, a two electron covalent bond is denoted by placing a line between the atoms. In a nitric acid molecule, there are twenty four valence electrons. The Lewis dot structure of nitric acid is given below:
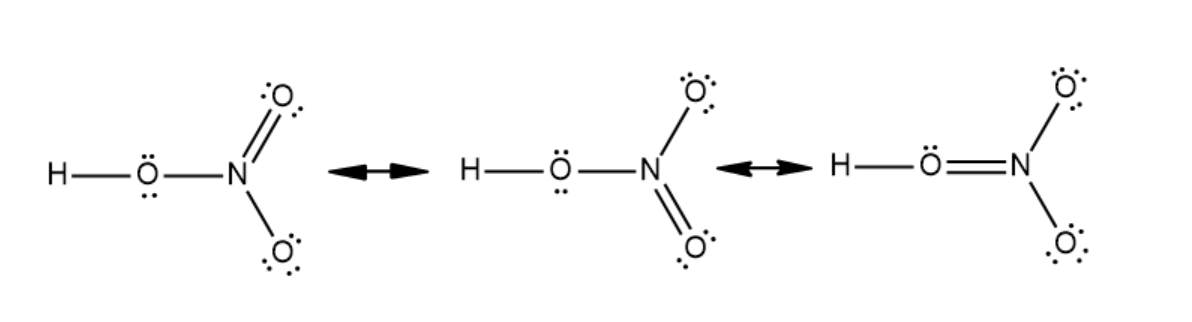
Note:
Remember that the Lewis structure which has zero formal charge or the structure in which formal charges are least separated is the preferred structure of the molecule. The octet rule has some exceptions in which the central atom is surrounded by less than eight electrons or sometimes more than eight electrons.
Complete answer:
The octet rule states that every atom binds with another atom either by donating electrons or accepting electrons in its valence shell. According to this rule, a complete atom should have eight electrons in its outermost shell. The atoms that do not have complete orbitals will accept electrons and complete their valence shell to fulfill the octet rule. This rule explains the formation of many compounds. It also explains the stability of various compounds. Lewis structure is a structure that shows a covalent bond as a pair of electrons divided among the two molecules. Adding the number of outermost electrons in all the atoms in molecules gives the total number of valence electrons in the molecule. In this structure, a two electron covalent bond is denoted by placing a line between the atoms. In a nitric acid molecule, there are twenty four valence electrons. The Lewis dot structure of nitric acid is given below:

Note:
Remember that the Lewis structure which has zero formal charge or the structure in which formal charges are least separated is the preferred structure of the molecule. The octet rule has some exceptions in which the central atom is surrounded by less than eight electrons or sometimes more than eight electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




