
State the IUPAC name of \[PhC{{H}_{2}}OH\].

Answer
586.2k+ views
Hint: IUPAC name is the universally accepted way of naming of a compound. To write the IUPAC name of the given compound, identify the name given to a benzene like ring with one less hydrogen. Then identify the group attached to it and determine if it’s primary or not. Put the two of the above mentioned together to find the final IUPAC name.
Complete step by step solution:
We know that IUPAC nomenclature is a method of naming chemical compounds as recommended by the International Union of Pure and Applied Chemistry.
There are certain steps that we have to follow while writing the IUPAC name of any compound.
-Firstly, we have to draw the structure. If it is a cyclic structure (as given to us in the question) we count the number of members in the ring. Do not forget to add the prefix –cyclo followed by the number of members i.e. prop, but, pent, hex etc.
-Then we have to check if it’s an –ane or –ene i.e. all the carbons are single bonded then –ane and if carbons double bonds then the for particular carbon (s) we use –ene.
-Next we have to look for the functional group present and then add the suffix accordingly. For example if we have an alcohol we end the name with –ol and if we have a ketone functional group we end it with –one etc.
Now, let us look at the given structure and try to find its IUPAC name.
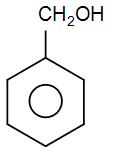
The compound given to us is –
Here, we can see we have a cyclic ring with a substituent. But, we do not have to use the term cyclo and –hex –ane/-ene here as we already have a name for such aromatic rings and that is phenyl.
We can see that we have substituent at only one carbon so we do not need to name the carbon atom; we can simply add the functional group name to the prefix phenol.
Here, we have a $C{{H}_{2}}OH$ group. We can see that it is a methyl group where one hydrogen atom is replaced by the –OH functional group. We know that the –OH functional group is present in alcohols. Therefore, the name of this particular substituent will be methyl alcohol which is also known as methanol.
So if we put the prefix and suffix together we get the IUPAC name as phenylmethanol.
Therefore, the IUPAC name of $PhC{{H}_{2}}OH$ is phenylmethanol and this is the required answer.
Note: The common name of the compound given to us is benzyl alcohol. We have named it as phenylmethanol and this is its preferred IUPAC name and it is also known as the PIN name. The systematic IUPAC name of benzyl alcohol is benzenemethanol. The phenyl ring is considered more or less as a benzene ring as the only difference between the two is that phenyl ring has one less hydrogen.
Complete step by step solution:
We know that IUPAC nomenclature is a method of naming chemical compounds as recommended by the International Union of Pure and Applied Chemistry.
There are certain steps that we have to follow while writing the IUPAC name of any compound.
-Firstly, we have to draw the structure. If it is a cyclic structure (as given to us in the question) we count the number of members in the ring. Do not forget to add the prefix –cyclo followed by the number of members i.e. prop, but, pent, hex etc.
-Then we have to check if it’s an –ane or –ene i.e. all the carbons are single bonded then –ane and if carbons double bonds then the for particular carbon (s) we use –ene.
-Next we have to look for the functional group present and then add the suffix accordingly. For example if we have an alcohol we end the name with –ol and if we have a ketone functional group we end it with –one etc.
Now, let us look at the given structure and try to find its IUPAC name.
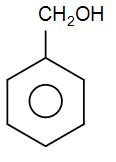
The compound given to us is –

Here, we can see we have a cyclic ring with a substituent. But, we do not have to use the term cyclo and –hex –ane/-ene here as we already have a name for such aromatic rings and that is phenyl.
We can see that we have substituent at only one carbon so we do not need to name the carbon atom; we can simply add the functional group name to the prefix phenol.
Here, we have a $C{{H}_{2}}OH$ group. We can see that it is a methyl group where one hydrogen atom is replaced by the –OH functional group. We know that the –OH functional group is present in alcohols. Therefore, the name of this particular substituent will be methyl alcohol which is also known as methanol.
So if we put the prefix and suffix together we get the IUPAC name as phenylmethanol.
Therefore, the IUPAC name of $PhC{{H}_{2}}OH$ is phenylmethanol and this is the required answer.
Note: The common name of the compound given to us is benzyl alcohol. We have named it as phenylmethanol and this is its preferred IUPAC name and it is also known as the PIN name. The systematic IUPAC name of benzyl alcohol is benzenemethanol. The phenyl ring is considered more or less as a benzene ring as the only difference between the two is that phenyl ring has one less hydrogen.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




