
State True or False.
${ O }_{ 2 }$, ${ O }_{ 2 }^{ - }$are paramagnetic while${ O }_{ 3 }$, ${ O }_{ 2 }^{ 2- }$are diamagnetic.
(a) True
(b) False
Answer
585k+ views
Hint: Draw the molecular orbital diagram , if all the electrons are paired, then it will be diamagnetic. On the other hand, if all of the electrons remain unpaired, then it will be diamagnetic.
Complete Step by Step answer:
${ O }_{ 2 }$, ${ O }_{ 2 }^{ - }$ are paramagnetic while${ O }_{ 3 }$, ${ O }_{ 2 }^{ 2- }$ are diamagnetic.
Structure of O2
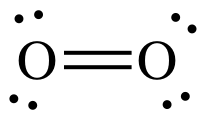
The given statement is True.
A molecule is said to be paramagnetic, if it has unpaired electrons present in it .
If all electrons are paired, then the molecule is said to be diamagnetic.
This is better explained by the molecular orbital diagram of ${ O }_{ 2 }$.
Since , all the compounds mentioned here are derivatives of${ O }_{ 2 }$, we can explain it by this diagram. In this diagram, the orbitals are arranged in the increasing order of energies.
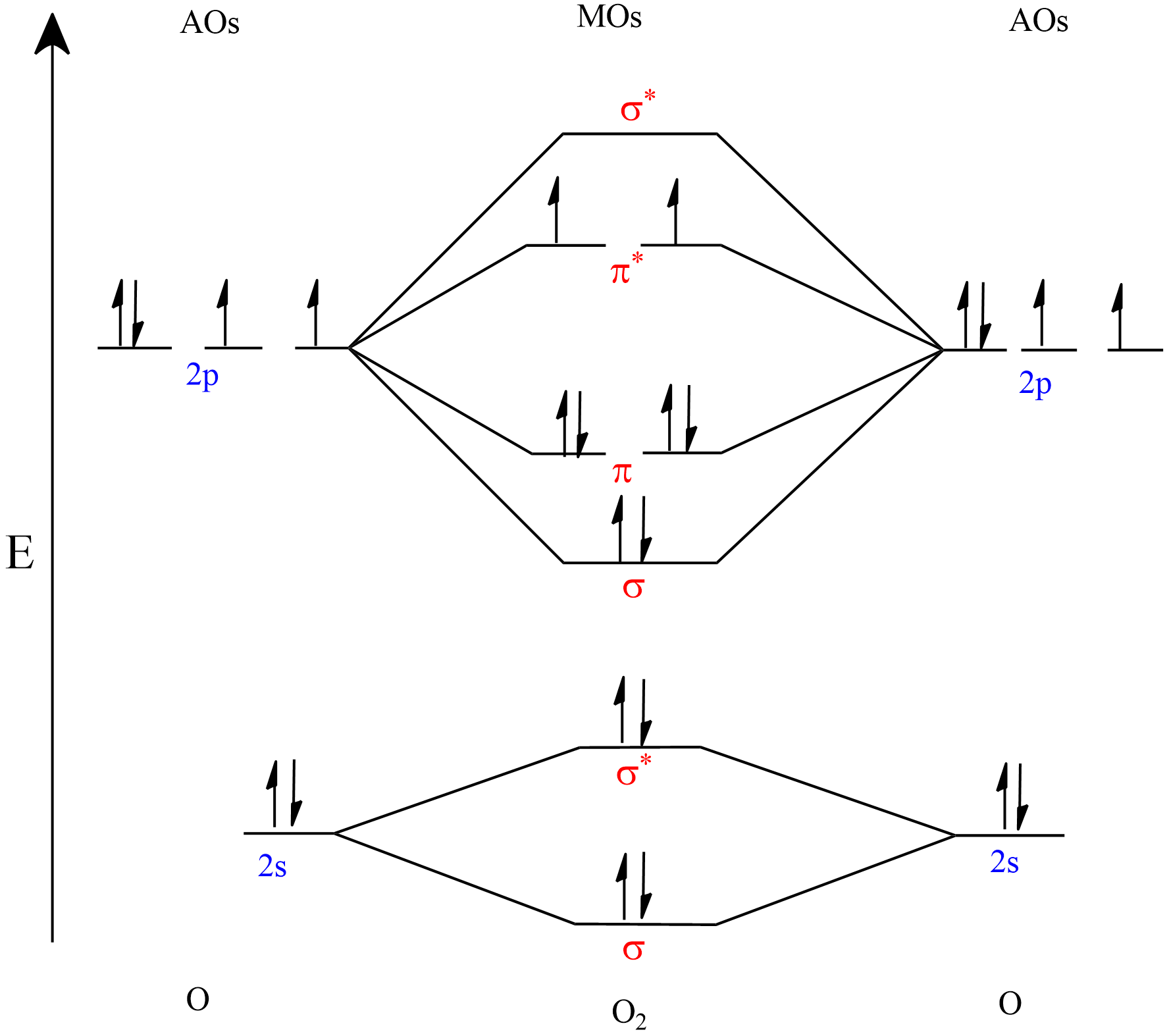
Here we can see that in${ O }_{ 2 }$, in the molecular orbital diagram, there are 2 unpaired electrons in the $\left( { \pi }^{ \ast }2p \right) $orbital. And hence it is paramagnetic.
In the case of ${ O }_{ 2 }^{ - }$, one more electron is added to the valence shell, and then that electron will enter into one of the $\left( { \pi }^{ \ast }2p \right) $orbitals. Then only one unpaired electron still exists. So, it is paramagnetic.
In the case of ${ O }_{ 2 }^{ 2- }$, two more electrons are added to the valence shell of ${ O }_{ 2 }$molecule, and then that two electrons will enter $\left( { \pi }^{ \ast }2p \right) $orbitals, then all the electrons will be paired. Hence, it is diamagnetic in nature.
In the case of ${ O }_{ 3 }$ also, all the electrons are paired, and hence it is diamagnetic.
So, the correct option is option (a) True.
Additional Information:
Paramagnetism : Paramagnetic substances are weakly attracted by a magnetic field. These are magnetized in a magnetic field in the same direction. They lose their magnetism due to the presence of one or more unpaired electrons which are attracted by the magnetic field.
Diamagnetism : Diamagnetic substances are weakly repelled by a magnetic field. These are weakly magnetized in a magnetic field in the opposite direction. Diamagnetism is shown by those substances in which all the electrons are paired and there are no unpaired electrons. Pairing of electrons cancels their magnetic moment and they lose their magnetic character.
Note: Also, one can explain also by using molecular orbital electronic configuration as well.
For Eg: In the case of ${ O }_{ 2 }$, the MO Electronic configuration is :
$\left[ He \right] { \sigma 2s }^{ 2 }{ \quad \sigma }^{ \ast }{ 2s }^{ 2 }{ \quad \pi 2 }{ p }_{ z }^{ 2 }{ \quad { \quad \pi 2 }{ p }_{ x }^{ 2 }\quad { \quad \pi 2 }{ p }_{ y }^{ 2 }\quad { \pi }^{ \ast }2 }{ p }_{ x }^{ 1 }\quad { \pi }^{ \ast }2{ p }_{ y }^{ 1 }$ , From this we can see that Oxygen is having 1 unpaired electrons each in ${ { \pi }^{ \ast }2 }{ p }_{ x }^{ 1 }\quad and\quad { \pi }^{ \ast }2{ p }_{ y }^{ 1 }$ and hence we can conclude that ${ O }_{ 2 }$ is paramagnetic, also one can determine in the case of other compounds using this method.
Complete Step by Step answer:
${ O }_{ 2 }$, ${ O }_{ 2 }^{ - }$ are paramagnetic while${ O }_{ 3 }$, ${ O }_{ 2 }^{ 2- }$ are diamagnetic.
Structure of O2
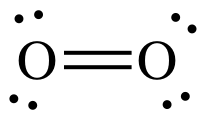
The given statement is True.
A molecule is said to be paramagnetic, if it has unpaired electrons present in it .
If all electrons are paired, then the molecule is said to be diamagnetic.
This is better explained by the molecular orbital diagram of ${ O }_{ 2 }$.
Since , all the compounds mentioned here are derivatives of${ O }_{ 2 }$, we can explain it by this diagram. In this diagram, the orbitals are arranged in the increasing order of energies.

Here we can see that in${ O }_{ 2 }$, in the molecular orbital diagram, there are 2 unpaired electrons in the $\left( { \pi }^{ \ast }2p \right) $orbital. And hence it is paramagnetic.
In the case of ${ O }_{ 2 }^{ - }$, one more electron is added to the valence shell, and then that electron will enter into one of the $\left( { \pi }^{ \ast }2p \right) $orbitals. Then only one unpaired electron still exists. So, it is paramagnetic.
In the case of ${ O }_{ 2 }^{ 2- }$, two more electrons are added to the valence shell of ${ O }_{ 2 }$molecule, and then that two electrons will enter $\left( { \pi }^{ \ast }2p \right) $orbitals, then all the electrons will be paired. Hence, it is diamagnetic in nature.
In the case of ${ O }_{ 3 }$ also, all the electrons are paired, and hence it is diamagnetic.
So, the correct option is option (a) True.
Additional Information:
Paramagnetism : Paramagnetic substances are weakly attracted by a magnetic field. These are magnetized in a magnetic field in the same direction. They lose their magnetism due to the presence of one or more unpaired electrons which are attracted by the magnetic field.
Diamagnetism : Diamagnetic substances are weakly repelled by a magnetic field. These are weakly magnetized in a magnetic field in the opposite direction. Diamagnetism is shown by those substances in which all the electrons are paired and there are no unpaired electrons. Pairing of electrons cancels their magnetic moment and they lose their magnetic character.
Note: Also, one can explain also by using molecular orbital electronic configuration as well.
For Eg: In the case of ${ O }_{ 2 }$, the MO Electronic configuration is :
$\left[ He \right] { \sigma 2s }^{ 2 }{ \quad \sigma }^{ \ast }{ 2s }^{ 2 }{ \quad \pi 2 }{ p }_{ z }^{ 2 }{ \quad { \quad \pi 2 }{ p }_{ x }^{ 2 }\quad { \quad \pi 2 }{ p }_{ y }^{ 2 }\quad { \pi }^{ \ast }2 }{ p }_{ x }^{ 1 }\quad { \pi }^{ \ast }2{ p }_{ y }^{ 1 }$ , From this we can see that Oxygen is having 1 unpaired electrons each in ${ { \pi }^{ \ast }2 }{ p }_{ x }^{ 1 }\quad and\quad { \pi }^{ \ast }2{ p }_{ y }^{ 1 }$ and hence we can conclude that ${ O }_{ 2 }$ is paramagnetic, also one can determine in the case of other compounds using this method.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




